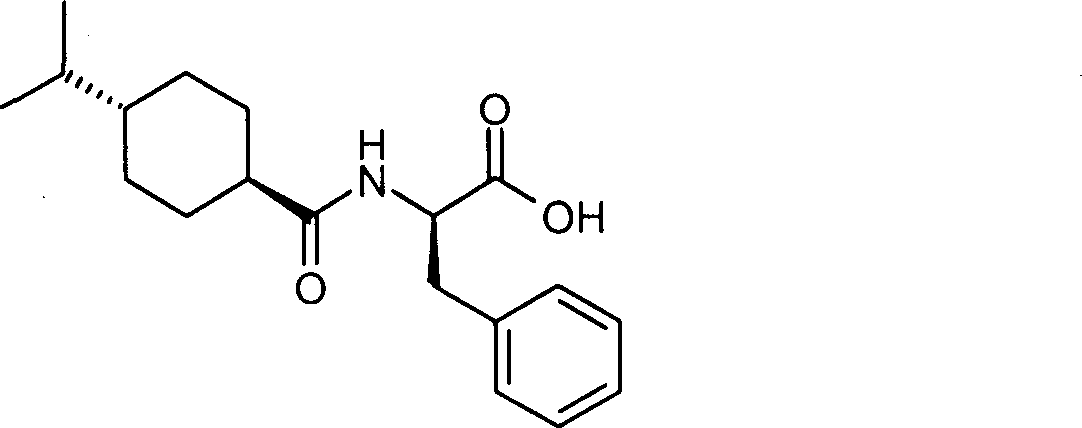
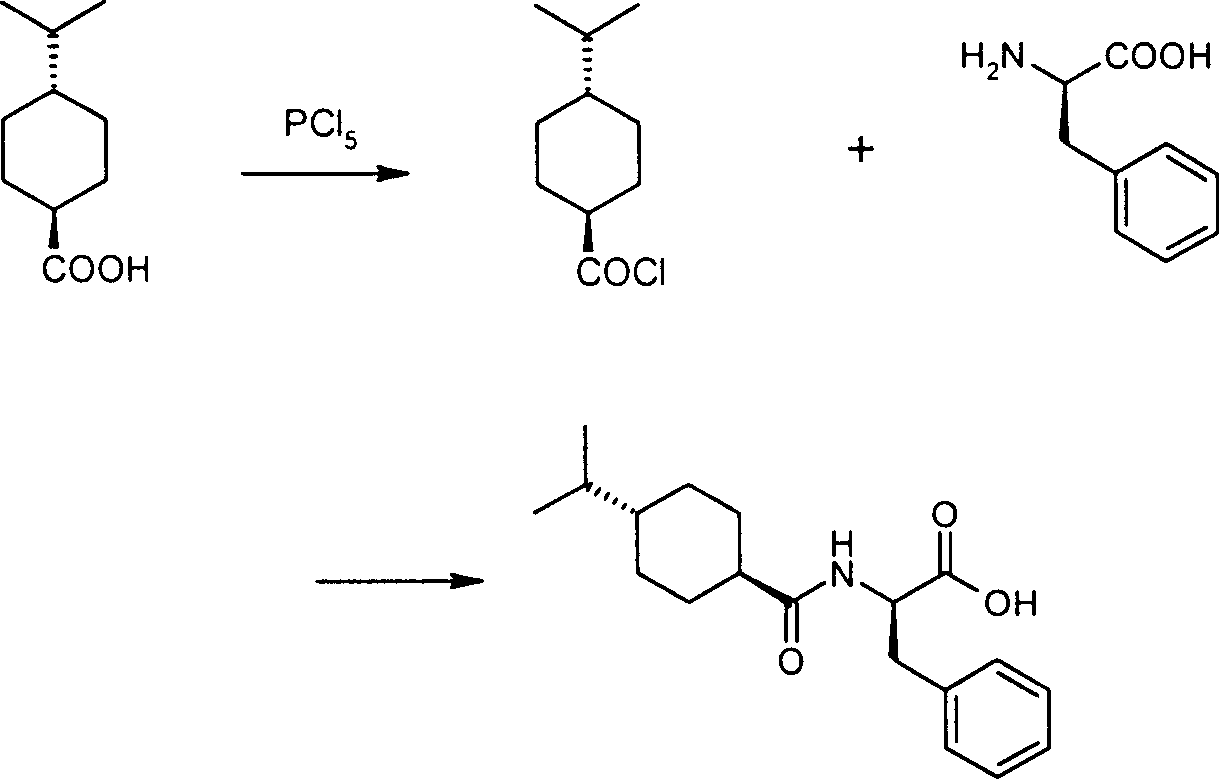
Improved preparation method of Nagelinei
A technology selected from hexamic acid, applied in the preparation of organic compounds, carboxylic acid amide preparation, chemical instruments and methods, etc., can solve the problems of incomplete reaction, difficulty in fully stirring, unsuitable for industrial production, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
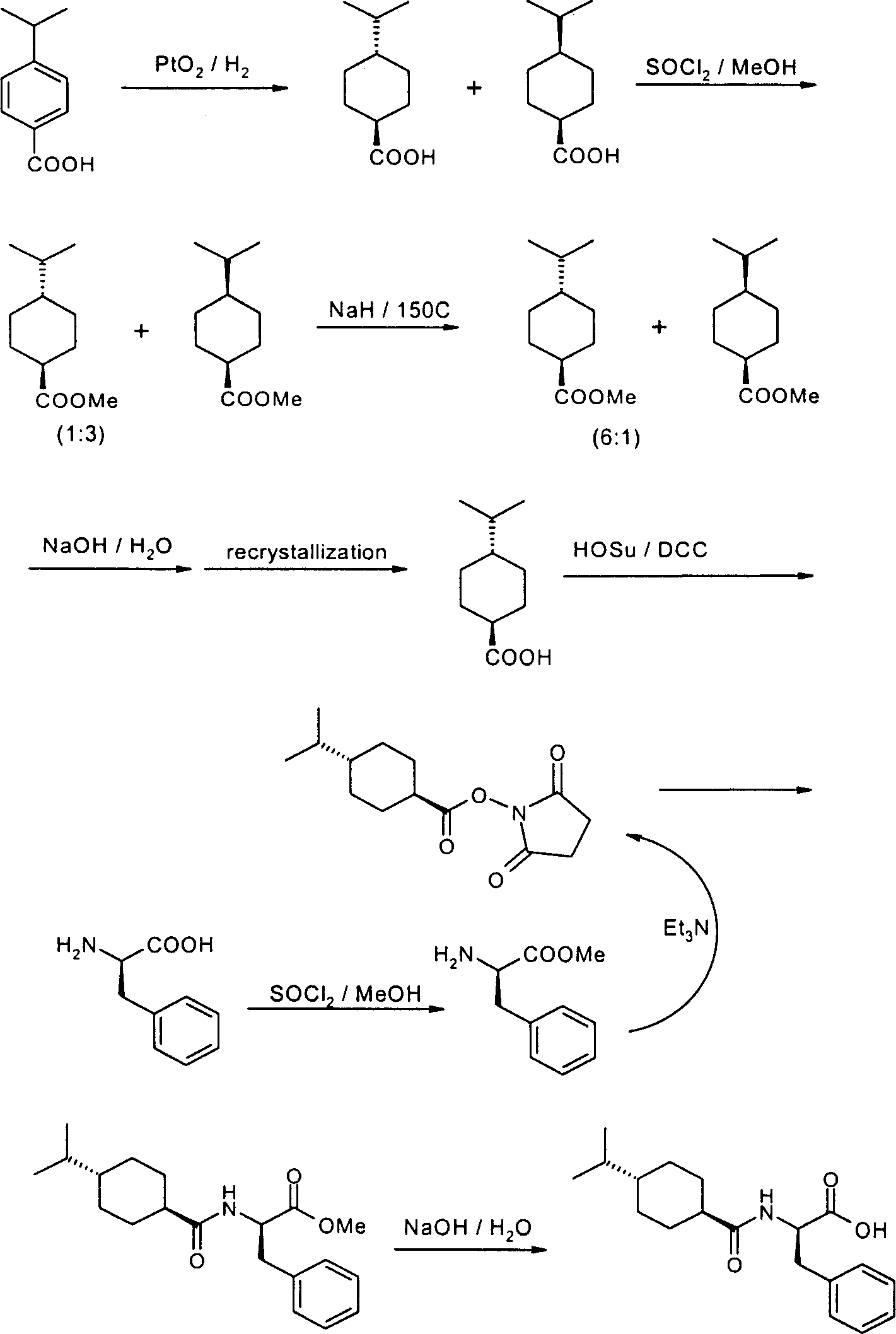
[0085] Embodiment one, the preparation of p-isopropylbenzoic acid (I)
[0086] Put the p-cymene raw material into a 1.0L autoclave and feed oxygen to the internal pressure of 22kg / cm 2 , heated to 105°C under stirring, and maintained under pressure for 2.5 hours. The reaction solution was filtered to remove solids, and the solvent was recovered under reduced pressure; the residue was poured into 500 ml of ice water, and a dark green precipitate appeared after stirring; the solids were collected by filtration and washed with water to obtain 65.0 g of a crude product. Recrystallized with glacial acetic acid-water to obtain 50.5 g of white crystal I, with a melting point of 118-119°C and a yield of 86%. Elemental analysis (C 10 h 12 o 2 =164.20): calculated value C73.15 H7.37; measured value C73.08 H7.37.
Embodiment 2
[0087] The preparation of embodiment two, 4-isopropyl-cyclohexanecarboxylic acid (II)
[0088] In a 1.0-liter autoclave, dissolve 50.0 grams of p-isopropylbenzoic acid in 500 milliliters of glacial acetic acid, then add 10.0 grams of 10% Pd-C, and feed hydrogen to 55.0 kg / cm 2 , heated to 150°C for 8 hours under stirring. After cooling, filter the reaction solution to recover the catalyst; distill the filtrate under reduced pressure to recover acetic acid. The residual oil was distilled at 126°C / 0.7mmHg to obtain 46.1 grams of a colorless transparent viscous liquid, namely 4-isopropyl
[0089] Mixture II of cis-trans isomers of cyclohexanecarboxylic acid, yield 89%.
Embodiment 3
[0090] Embodiment three, the preparation of trans-4-isopropyl cyclohexanecarboxylic acid (III)
[0091] Take 46.0 g of the above mixture II, dissolve it in 150 ml of p-cymene, add 37.0 g of solid potassium hydroxide (content not less than 82%), heat to 145° C. under nitrogen protection, and stir for 6 hours. After the reaction, cool down; add a mixed solution of 60 ml of water and 60 ml of methanol for extraction, separate the lower layer (water / alcohol phase) after standing for stratification, acidify to pH 2 with concentrated hydrochloric acid in an ice bath; stir at low temperature for 1 hour, and filter Precipitate and recrystallize twice with formic acid (content not less than 85%) to obtain 37.0 g of white crystal III, melting point: 94-95°C, yield 80%. The HPLC analysis content was greater than 99%, and no cis-isomer was detected. Elemental analysis (C 10 h 18 o 2 =170.25): calculated value (%) C70.55 H10.66; measured value (%) C70.57 H10.84.
[0092] HPLC instrume...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com