Use of dipolymer of phthalide
A technology of anti-tumor drugs and compositions, which is applied in the field of preparation of anti-tumor drug compositions, can solve the problems of limited clinical application, toxic and side effects, etc., and achieve enhanced killing of tumor cells, fast effect, and multi-drug resistance sex-reducing effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] Example 1: Isolation and identification of LA
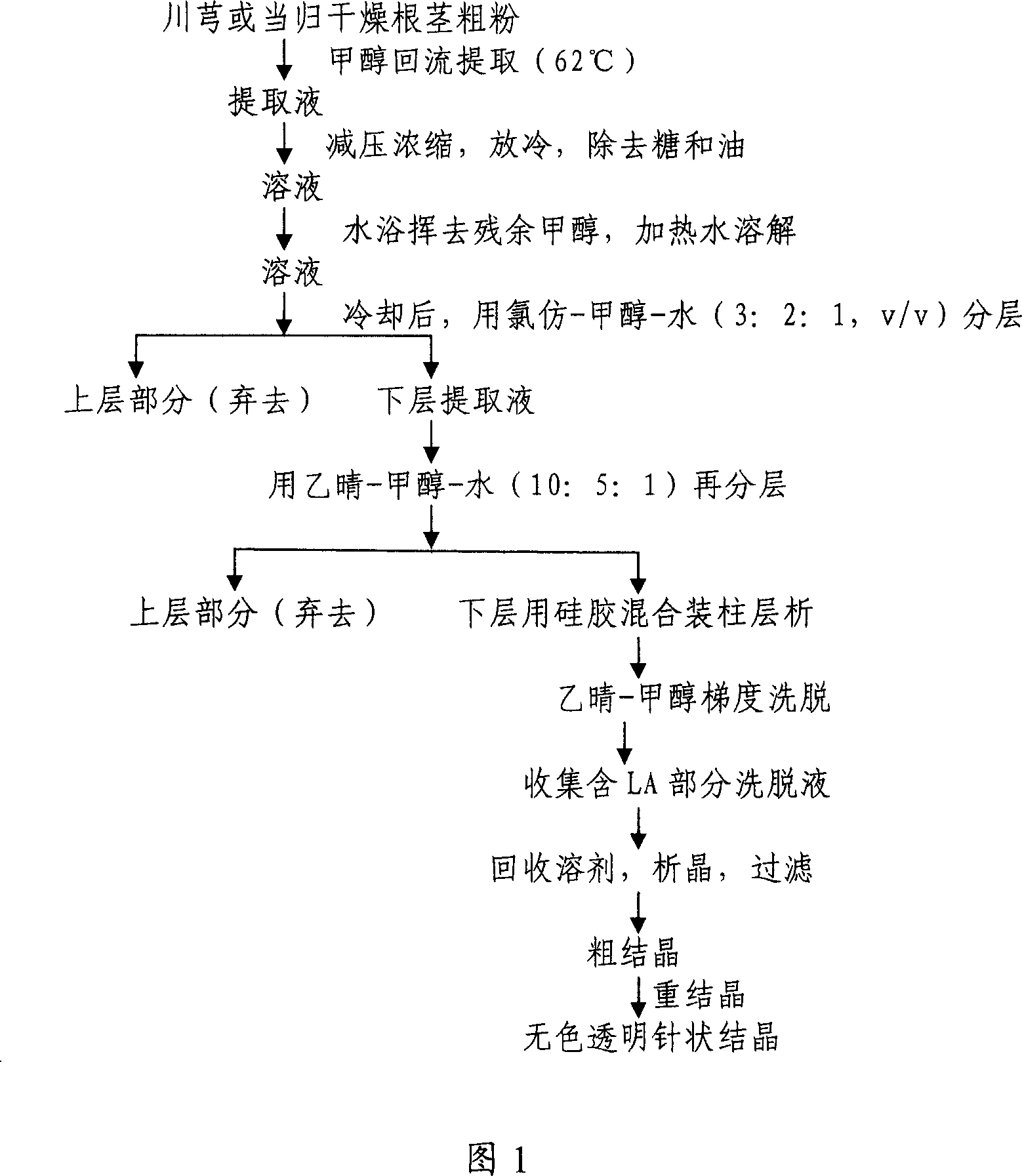
[0035] The process steps for isolating and purifying the compound LA from Chuanxiong or Angelica sinensis can be seen in the flow chart of FIG. 1 .
[0036] The separation method of LA can be found in the articles of wang PS et al. (Phytochemistry 1984; 23; 2033-8 and Chinese Pharmaceutical Industry 1988; 19:553-9) or Kaouadji M et al. (J Nat Prod 1986; 49:872-7) and Naito Two articles by T et al. (Heterocycles 1991; 32: 2433-42).
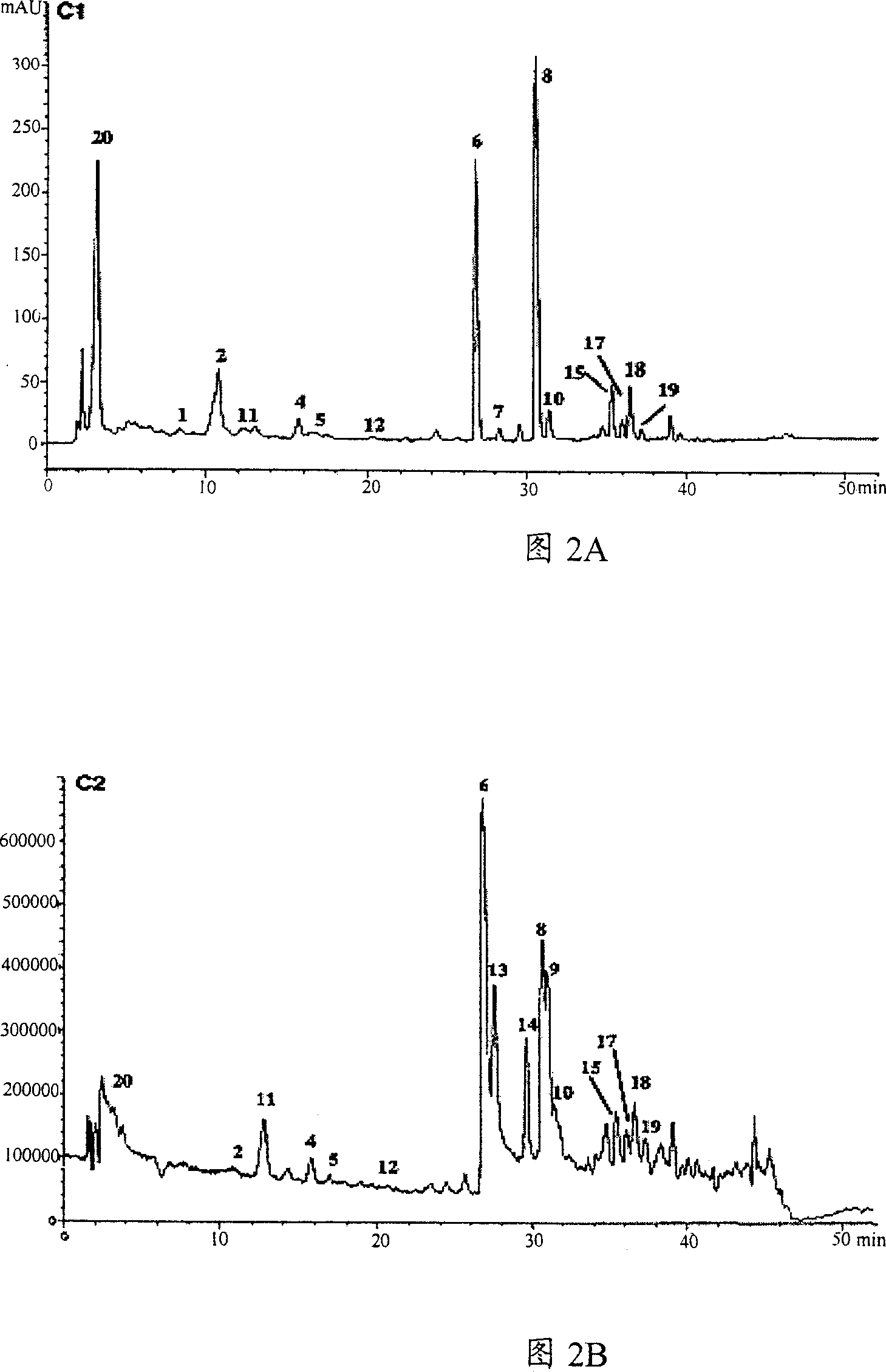
[0037] The material obtained from Ligusticum chuanxiong according to the process steps in Fig. 1 in the present invention is a colorless transparent crystal, which is identified by LC-MS and HNMR. The LC-MS real-time chromatographic data of Chuanxiong extract at 294nm are shown in Figure 2A and Figure 2B.
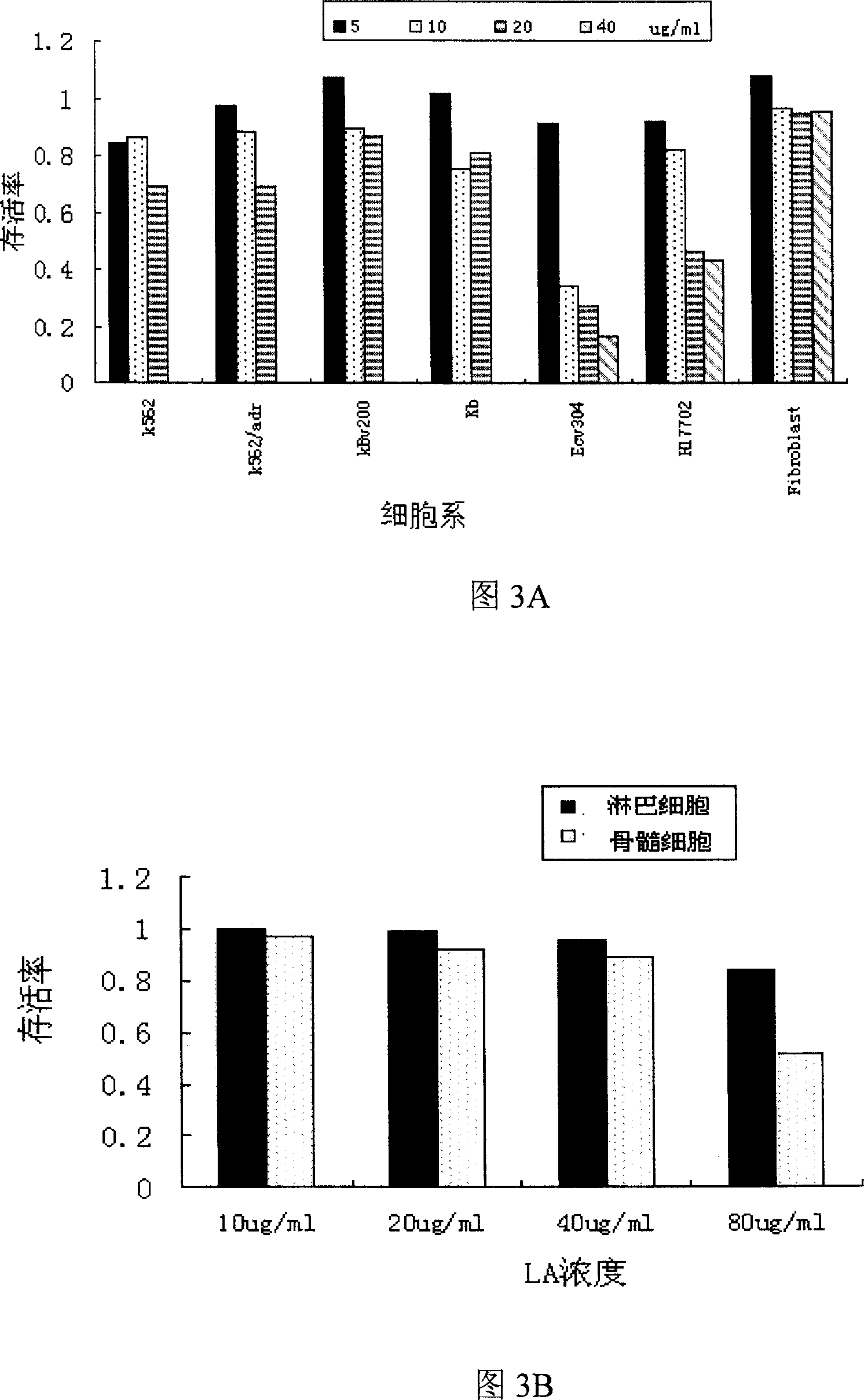
[0038] Embodiment 2-7 is to the cytological utility evaluation of compound LA
[0039] Among them, the cell lines used are k562 (human chronic myelogenous leukemia cell line) and k562 / adr...
Embodiment 2
[0044] Example 2: Measuring the main drug resistance mechanism of each drug-resistant strain
[0045] Collect cultured 5×10 6 K562, k562 / adr, kb, kbv200 cells were washed twice with PBS, followed by the instructions of the P-gp fluorescent antibody kit (UIU2), and FACS was performed to detect the expression of P-gp, and 10,000 cells were counted for each sample.
[0046] The determination results of the main drug resistance mechanism of each drug-resistant strain:
[0047] k562: P-gp expression 1.02%; k562 / adr: P-gp high expression up to 83.6%;
[0048]kb: P-pg expression was 0.87%; kbv200: P-gp was highly expressed to 76.3%.
[0049] The above experiments proved that the main drug resistance mechanism of the two drug-resistant cell lines k562 / adr and kbv200 was overexpression of P-pg, while the expression of p-gp in the sensitive strains was negative.
Embodiment 3
[0050] Embodiment 3: Measuring the multiples of drug resistance of each drug-resistant strain to doxorubicin and vincristine
[0051] 1).k562 / adr cell line
[0052] Take cells in the logarithmic growth phase, use RPMI1640 culture medium containing 10% calf serum to make cell suspension, add 100ul per well to a 96-well plastic culture plate, so that the number of cells per well is 2×10 4 After 30 minutes, add 100ul chemotherapeutic drugs doxorubicin and vincristine according to the concentration gradient of 0, 0.01, 0.1, 0.5, 1, 5, 10, and 20ug / ml respectively. The volume is 200ul, and the insufficient part is made up with RPMI 1640, placed in a carbon dioxide incubator, 37°C, saturated humidity, 5% CO 2 cultured under the condition of CO 2 conditions for 68 hours. Add 50ul of MTT (2mg / ml) to each well to remove the supernatant, add 120ul of DMSO to each well, oscillate with a micro-oscillator to fully dissolve the drug, and detect the OD value at 590nm wavelength by Elisa. ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| hydrophobicity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com