Method for dechloridizing organochloric compound
A compound and organochlorine technology, applied in the field of dechlorination of organochlorine compounds, can solve problems such as long-distance global migration, and achieve the effect of low reaction temperature
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
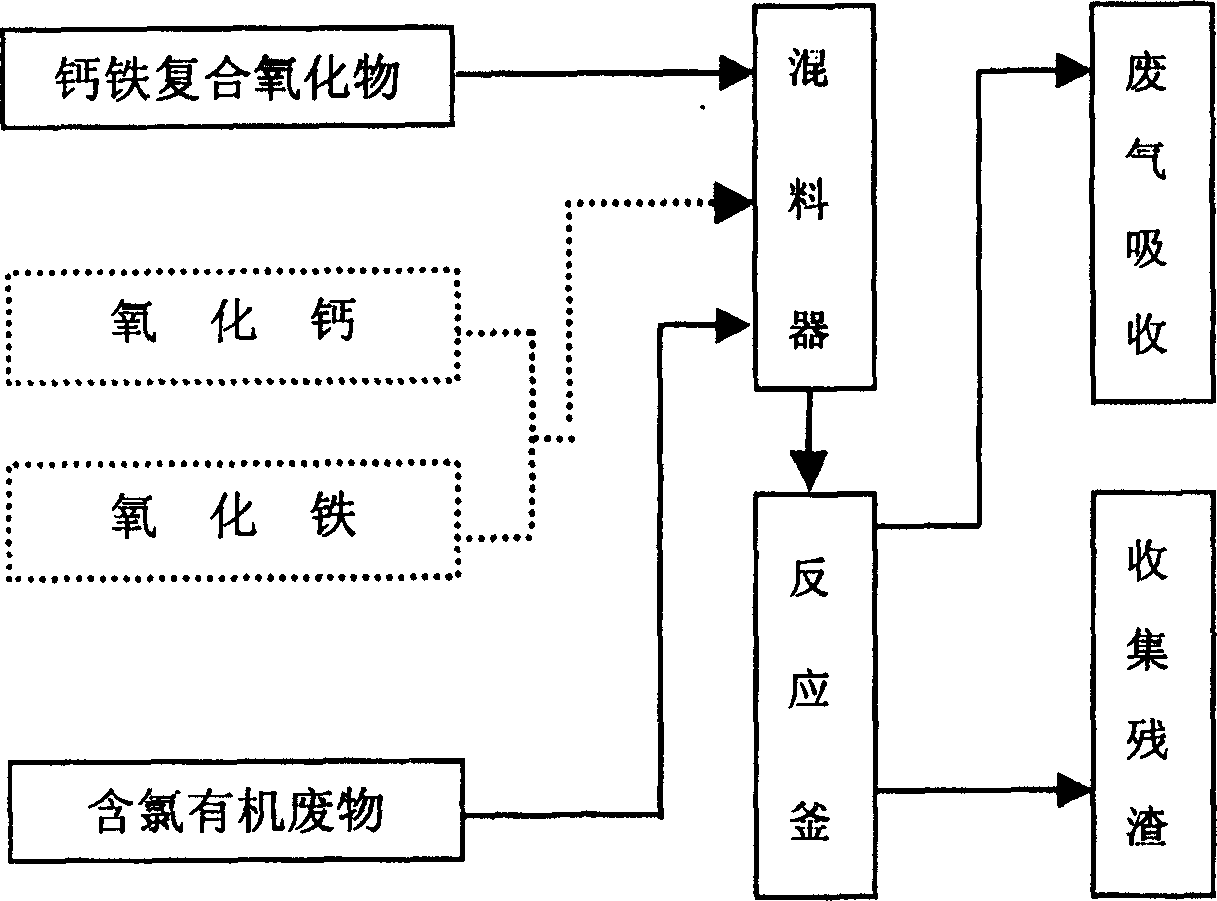
[0026] Calcium oxide and iron oxide are ground, sieved and dried. Put 2mg of hexachlorobenzene, 100mg of calcium oxide and 100mg of iron oxide mixture on 1.38cm 3Mix well in an airtight container and react at 300°C for 1 hour. The residue after the reaction was extracted with n-hexane, and the organic phase was extracted with pure water to wash away the water-soluble impurities. The obtained n-hexane solution was detected by GC / MS to analyze the organic products after the dechlorination reaction, indicating that hexachlorobenzene had been eliminated. Completely dechlorinated. After the remaining solid matter was dried, the inorganic compounds were analyzed by X-ray diffraction, which showed that the residues were CaO, Fe 2 o 3 , CaCl 2 , CaCO 3 Wait.
Embodiment 2
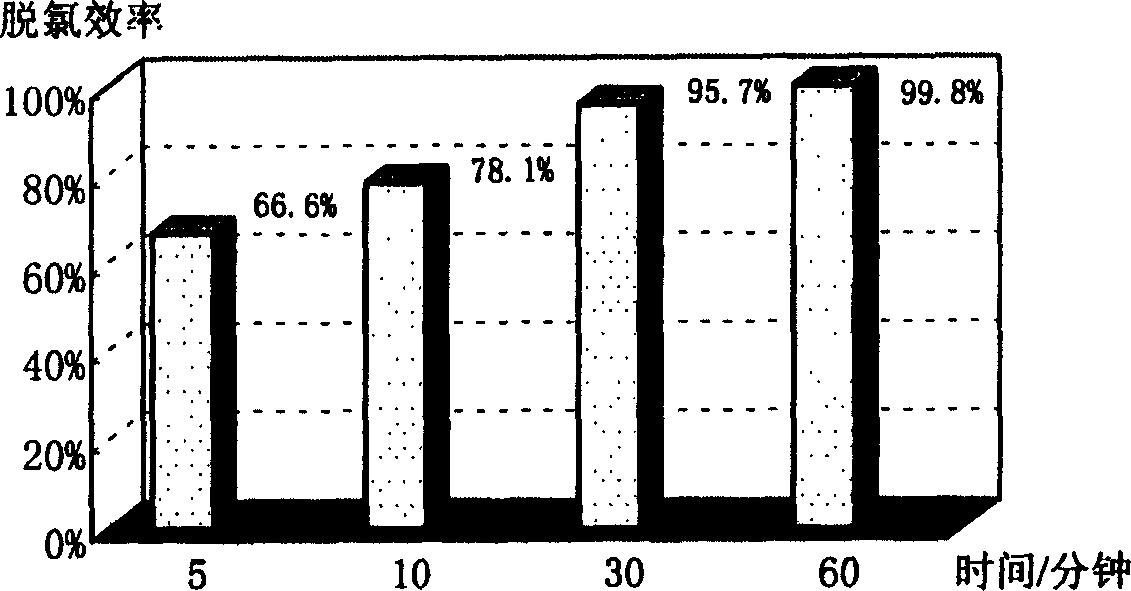
[0028] Calcium oxide and iron oxide are ground, sieved and dried. Put 2mg of hexachlorobenzene, 100mg of calcium oxide and 100mg of iron oxide mixture on 1.38cm 3 Mix well in a closed container, and react at 300°C. The reaction times are 5 minutes, 10 minutes, 30 minutes, and 60 minutes, respectively. The residue after the reaction was extracted with n-hexane, and the organic phase was extracted with pure water to wash away the water-soluble impurities, and the obtained n-hexane solution was detected by GC / MS to analyze the organic products after the dechlorination reaction. After analysis and calculation, the dechlorination efficiencies at 5 minutes, 10 minutes, 30 minutes and 60 minutes are respectively 66.6%, 78.1%, 95.7% and 99.8%. The relationship between dechlorination efficiency and reaction time is as follows: figure 1 shown.
Embodiment 3
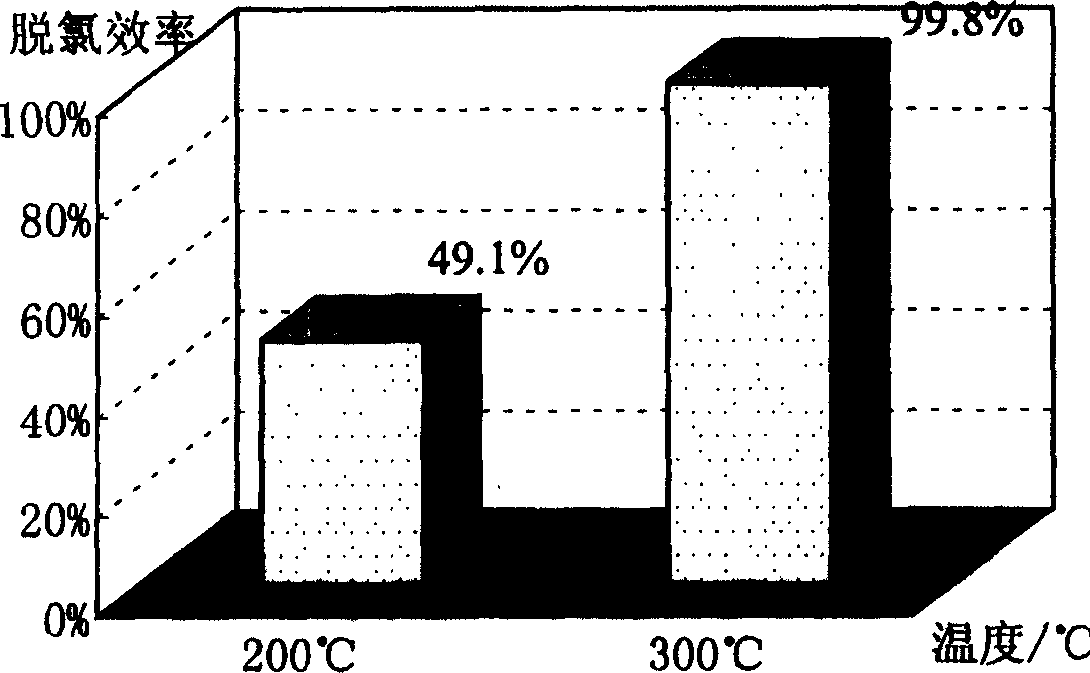
[0030] Calcium oxide and iron oxide are ground, sieved and dried. Put 2mg of hexachlorobenzene, 100mg of calcium oxide and 100mg of iron oxide mixture on 1.38cm 3 Mix well in a closed container, react at 200°C and 300°C for one hour respectively, extract the residue after the reaction with n-hexane, extract the organic phase with pure water, wash away the impurities soluble in water, and obtain n-hexane solution The organic products after the dechlorination reaction were detected and analyzed by GC / MS, and the dechlorination efficiencies at 200°C and 300°C were respectively 49.1% and 99.8% through analysis and calculation. The relationship between dechlorination efficiency and reaction temperature is as follows: figure 2 shown.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com