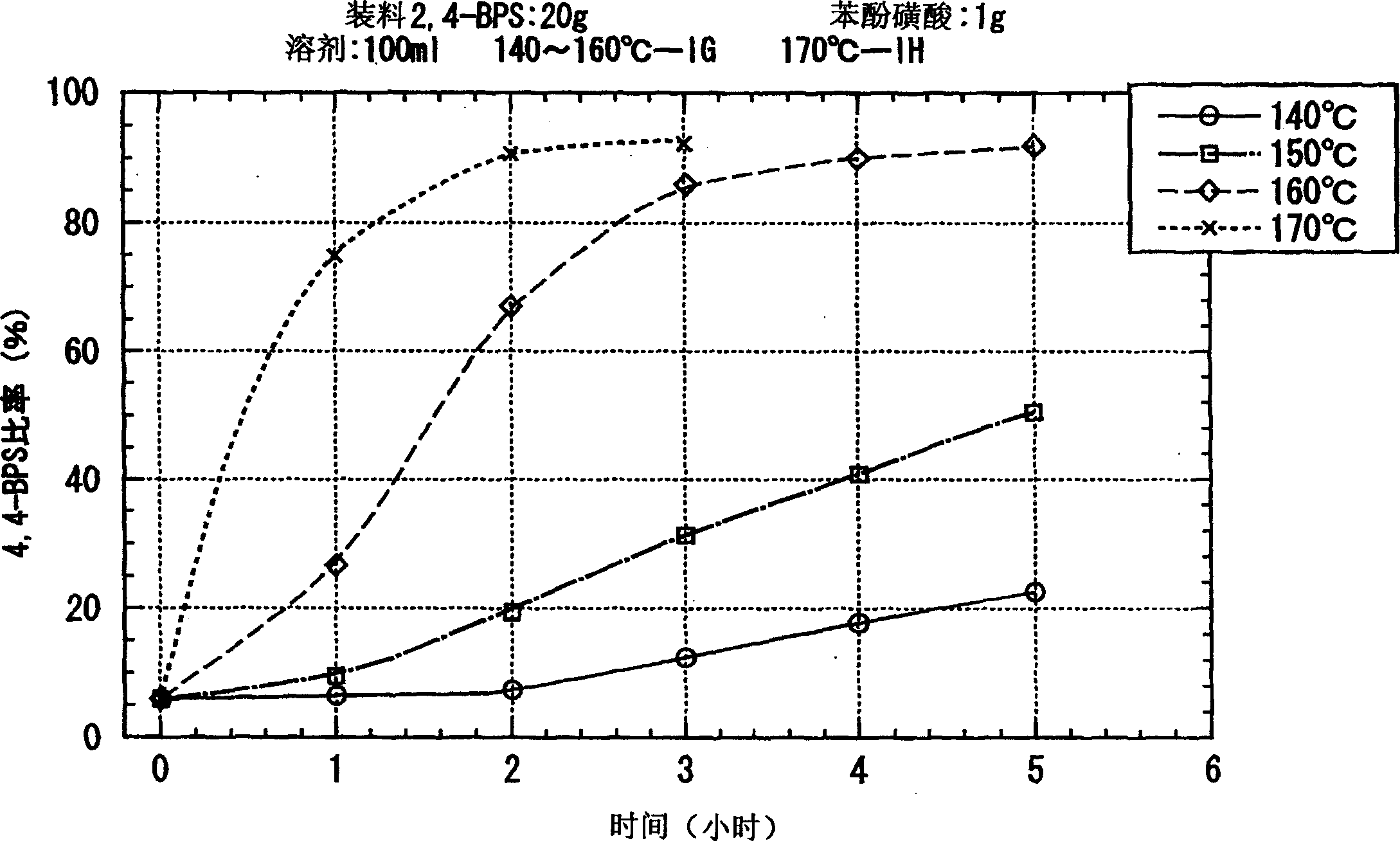
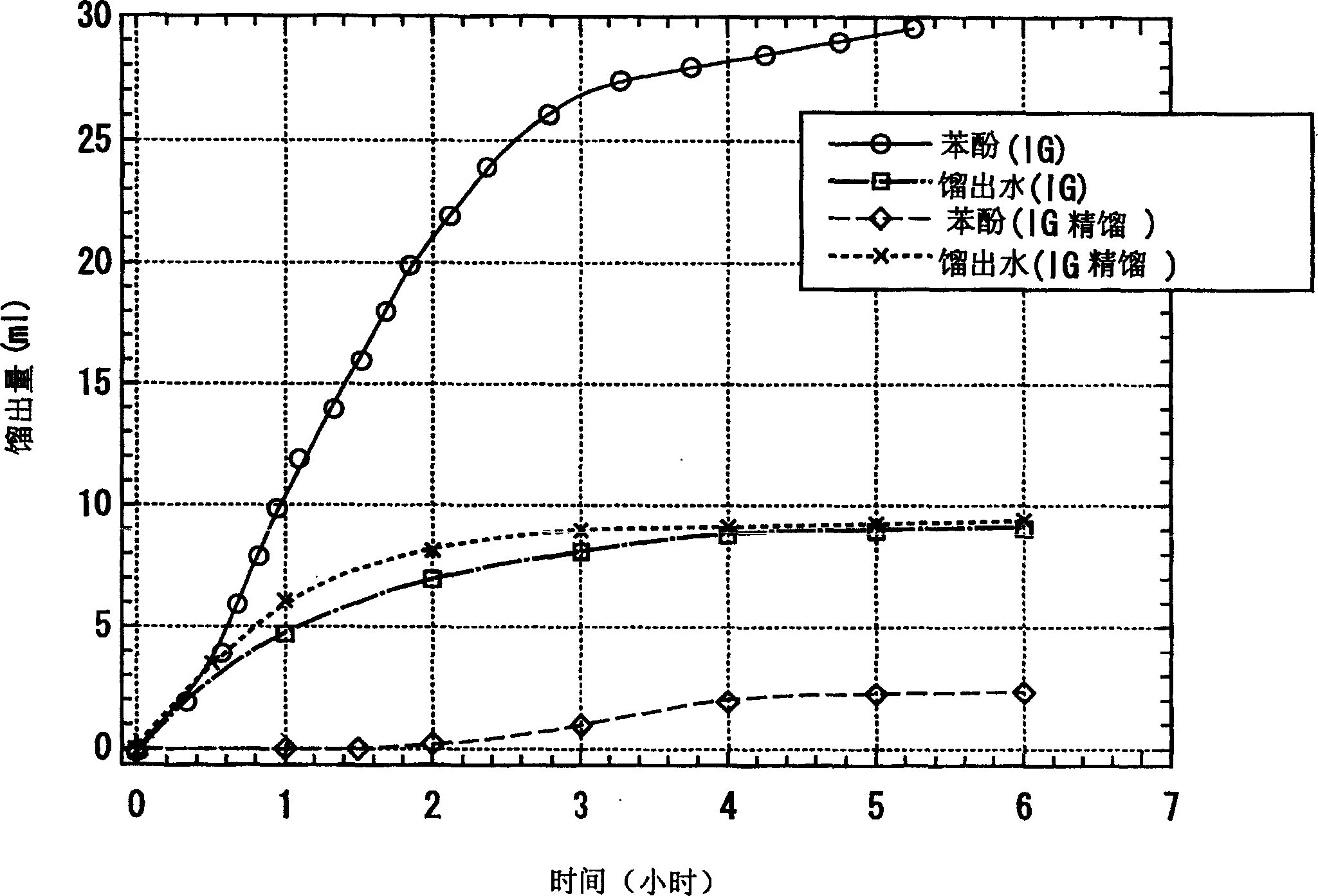
Process for producing 4, 4'-bisphenol sulfone
A manufacturing method, phenol sulfone technology, applied in the direction of chemical instruments and methods, preparation of organic compounds, organic chemistry, etc., can solve problems such as difficult to handle solid block, no post-processing operation advantage, difficulty, etc., to achieve simple reflux operation, Easy to recycle and reuse, and the effect of promoting the removal of water
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0101] Put 98.7g (1.05mol) of phenol, 75ml of Aisopa-H, and 50ml of mesitylene into a 500ml four-neck flask equipped with a stirrer, a thermometer, and a water separation tube. 51.6 g (0.50 mol) of 95% sulfuric acid was added dropwise thereto, and then the temperature was raised.
[0102] Distillation of the reaction liquid started from around 148° C., the distillate was condensed in a water separation tube and separated into two layers, and the upper organic layer was continuously returned to the reaction system. The temperature of the reaction system reached 170° C. after about 4 hours from the start of the temperature rise, the generation of water stopped, and the amount of water separated in the water separation tube was 20.5 g. Thereafter, 50 ml of Asopa-H was added, and it took 3 hours for the temperature of the reaction system to reach 175° C. to distill off mesitylene. The composition of the reactant was analyzed by high-performance liquid chromatography (HPLC), and t...
Embodiment 2
[0106] 98.7 g (1.05 moles) of phenol and 100 ml of Aisopa-G were charged into a 500 ml four-necked flask equipped with a stirrer, a thermometer, and a water separation tube, and 95% sulfuric acid 51.6 g was added dropwise to the mixture at 50°C under stirring conditions. g (0.50 mol), the temperature was raised.
[0107] Distillation of the reaction liquid started at around 144°C, and the distillate was condensed and separated into three layers in a water separation tube. The upper layer was a solvent layer, the middle layer was a water layer, and the bottom layer was a phenol layer. The upper solvent layer was continuously returned to the reaction system, and the bottom phenol layer was extracted every 15 minutes and returned to the reaction system. The temperature of the reaction system reached 167° C. in about 7 hours from the start of the temperature rise, the generation of water stopped, and the amount of water separated in the water separation tube was 21.2 g. The total...
Embodiment 3
[0111] Put 117.5 g (1.25 moles) of phenol and 100 ml of Aisopa-G into a 500 ml four-necked flask equipped with a stirrer, a thermometer, a water separation tube, and a Wedman-type rectification column, and add phenol to it at 50 ° C under stirring conditions. After 51.6 g (0.50 mol) of 95% sulfuric acid was added dropwise to the mixture, the temperature was raised.
[0112] Distillation of the reaction liquid started around 144°C, and the distillate was condensed and separated into three layers in the water separation tube, the upper layer being a solvent layer, the middle layer being a water layer, and the bottom layer being a phenol layer. The upper solvent layer was continuously returned to the reaction system, and the bottom phenol layer was reacted without returning to the reaction system. The temperature of the reaction system reached 167° C. in about 10 hours from the start of the temperature rise, and the generation of water stopped. The amount of water separated in th...
PUM
| Property | Measurement | Unit |
|---|---|---|
| boiling point | aaaaa | aaaaa |
| boiling point | aaaaa | aaaaa |
| boiling point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com