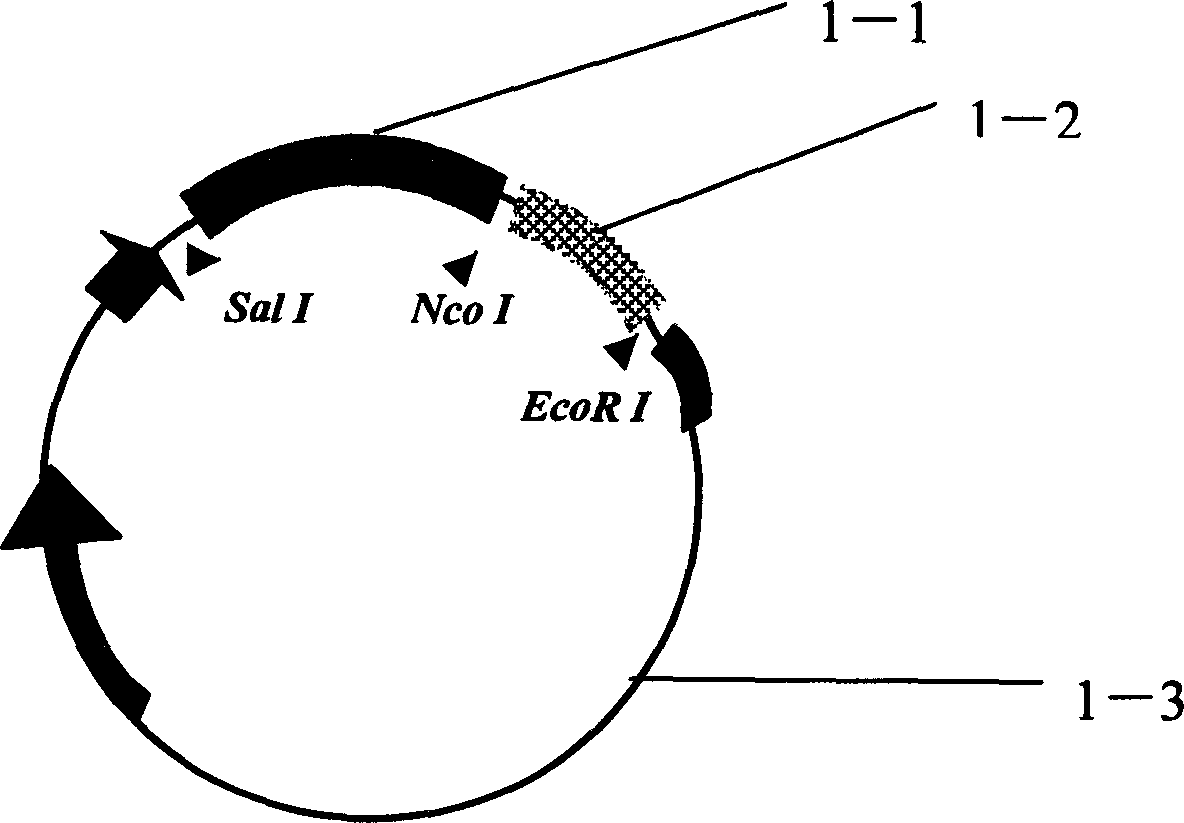
Plasmid of recombinant immunotoxin Rantes-DT390 aimed at activating Th1 cell, and its preparing method and use
An immunotoxin and recombinant plasmid technology, applied in biochemical equipment and methods, recombinant DNA technology, chemical instruments and methods, etc., can solve the problems of infection, high technical difficulty, low specificity, etc., to reduce non-specific binding, reduce Toxic and side effects, the effect of reducing adverse reactions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] Example 1: Preparation of recombinant immunotoxin eukaryotic plasmid
[0025] The vector used was the SRα eukaryotic plasmid containing DT390 (provided by PhD. Hu Huaizhong, University of Wisconsin, USA, Invitrogen Company), and the specific steps were as follows:
[0026] 1. Pretreatment of mouse Rantes gene (the following reagents were purchased from Promega Company)
[0027] (1) Use Trizol reagent to extract mouse liver total RNA according to the following steps
[0028] 1) Mouse liver tissue 100mg.
[0029] 2) Add 1ml Trizol.
[0030] 3) homogenate.
[0031] The homogenate should be thorough, and then transferred to EP tubes. When the amount of tissue homogenate is > 100mg, it will be divided into 1ml / each EP tube.
[0032] 4) Inverted and mixed for 10 times, room temperature for 5 minutes.
[0033] 5) Add 1 / 5 volume of chloroform (0.2ml, must be 1 / 5 of the total volume).
[0034] 6) Inverted and mixed for 10 times, room temperature for 5 minutes.
[0035] 7) C...
Embodiment 2
[0105] Example 2: Determination of biological activity of recombinant immunotoxin Rantes-DT390 in vitro
[0106] 1. Protein synthesis inhibition test
[0107] (1) Take the spleen of the mouse on the aseptic operating table, obtain the spleen cell suspension, and inoculate it in a cell culture bottle (about 10×10 6 cells / ml), add conA (10ug / ml) for mixed culture;
[0108] (2) After culturing for 3 days, harvest the cells, count and adjust the cell concentration to 1×10 6 cells / ml;
[0109] (3) Transfer to a 96-well cell culture plate (the culture medium is DMEM medium without leucine), and the above-mentioned transfection supernatants were divided into 1 / 2, 1 / 4, 1 / 8, 1 / 20, Add 1 / 50 diluted concentration, 50 μl / well, and set up triplicate wells for each concentration;
[0110] (4) Continue culturing for 24 hours, add 10 μl of 3H leucine (5 μci / ml), collect cells after 2 hours, perform liquid scintillation assay and calculate protein synthesis inhibition rate (relevant result...
Embodiment 3
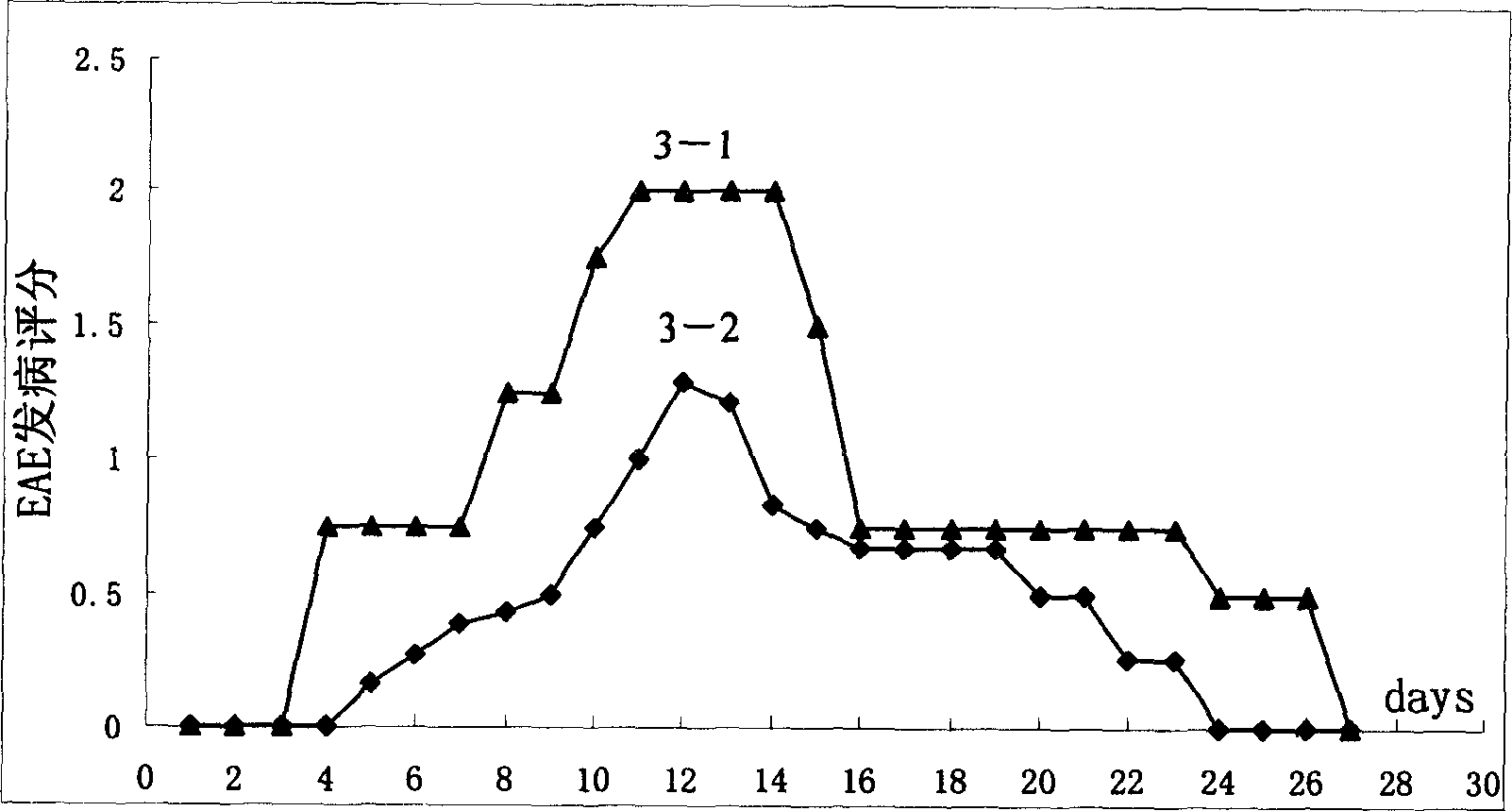
[0116] Example 3: Preliminary therapeutic effect of recombinant immunotoxin Rantes-DT390 eukaryotic plasmid on autoimmune disease animal model EAE (experimental allergic encephalomyelitis)
[0117] 1. Establishment of EAE (Experimental Allergic Encephalomyelitis) animal model: The EAE model was established with C57BL / 6 mice according to conventional methods.
[0118] (1) Use C57BL / 6 mice to establish an EAE animal model, mix the self-extracted MBP crude extract with FCA (containing Mycobacterium tuberculosis 5mg / ml) in equal volumes, use a 3ml syringe to repeatedly push and pull to form a water-in-oil emulsion , by intraperitoneal injection.
[0119] (2) Immunization dose: each mouse was injected with 0.4ml of MBP: FCA (1:1) mixed solution, 0.2ml of pertussis bacilli liquid: PBS (1:50) mixed solution (containing 0.6-1.8×106 bacillus pertussis) ).
[0120] (3) In the control group, each mouse was intraperitoneally injected with 0.2 ml of a mixture of Bacillus pertussis liquid...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com