Method for assaying compounds or agents for ability to decrease the activity of microsomal prostaglandin E synthase or hematopoietic prostaglandin D synthase
A prostaglandin and compound technology, applied in the screening of compounds, chemical treatment to inactivate enzymes, biochemical equipment and methods, etc., can solve the problems of undetectable fluorescence emission and achieve high-throughput effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0076]The preparation of combinatorial compound libraries is well known to those of ordinary skill in the art. Such combinatorial compound libraries include, but are not limited to, peptide libraries (see, e.g., U.S. Pat. -88). The synthesis of peptides is by no means the only method foreseen and contemplated for use in the present invention. Other chemistries that generate chemical multiplex libraries can also be used. Such chemical substances include, but are not limited to: peptoids (PCT publication WO 91 / 19735, December 26, 1991), encoded peptides (PCT publication WO 93 / 20242, October 14, 1993), Japan), random bio-oligomers (PCT Publication WO 92 / 00091, January 9, 1992), benzodiazepines (U.S. Patent No. 5,288,514), diversomers such as hydantoins Classes, benzodiazepines and dipeptides, etc. (Hobbs et al., (1993) Proc.Nat.Acad.Sci.USA 90: 69096913), vinylogous polypeptides (vinylogouspolypeptides) (Hagihara et al. (1992) J.Amer.Chem. Soc.114:6568), non-peptidic peptidom...
Embodiment I
[0083] Fluorescence Polarization Assay of Inducible Microsomal Prostaglandin E Synthase (mPGES)
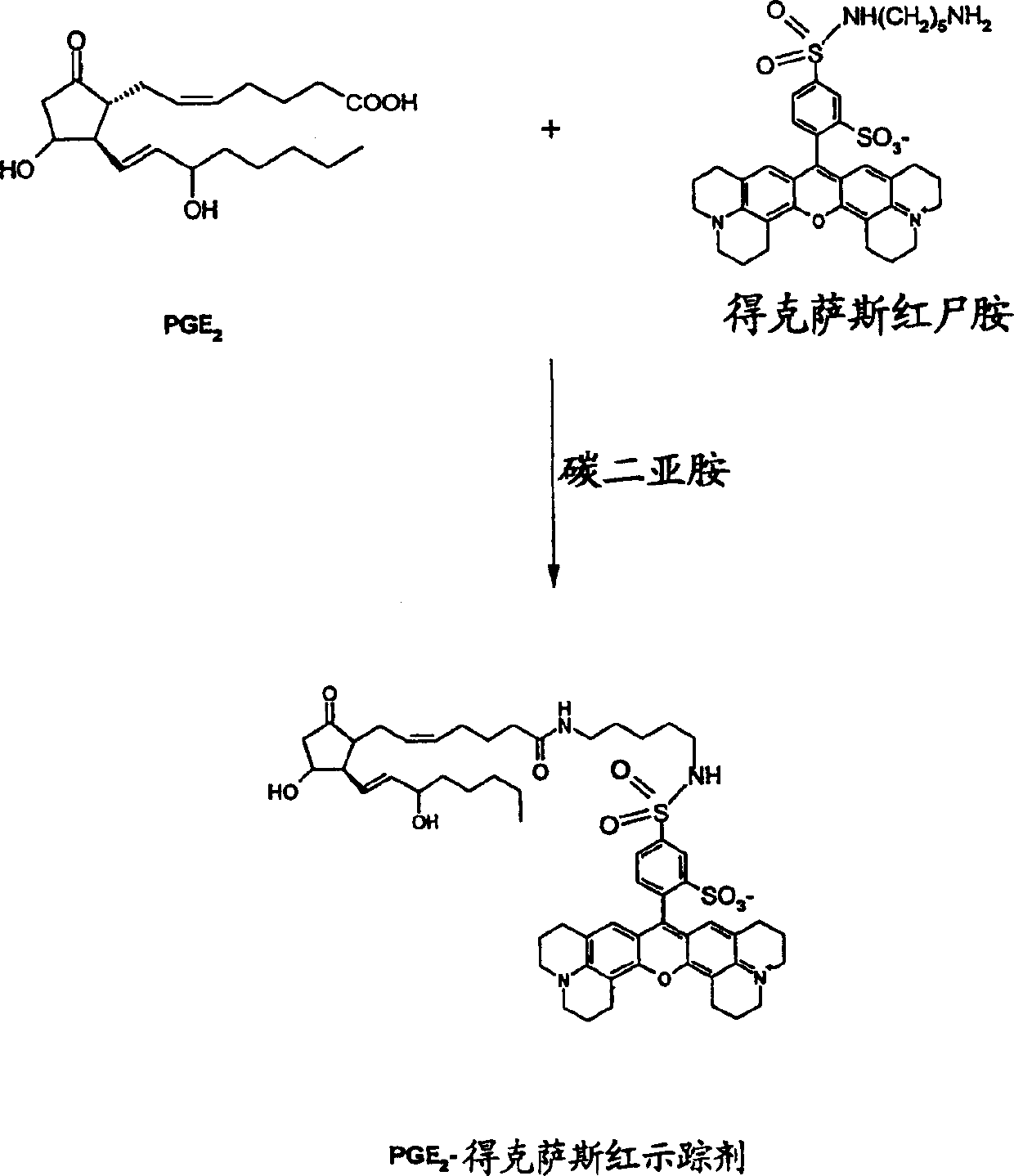
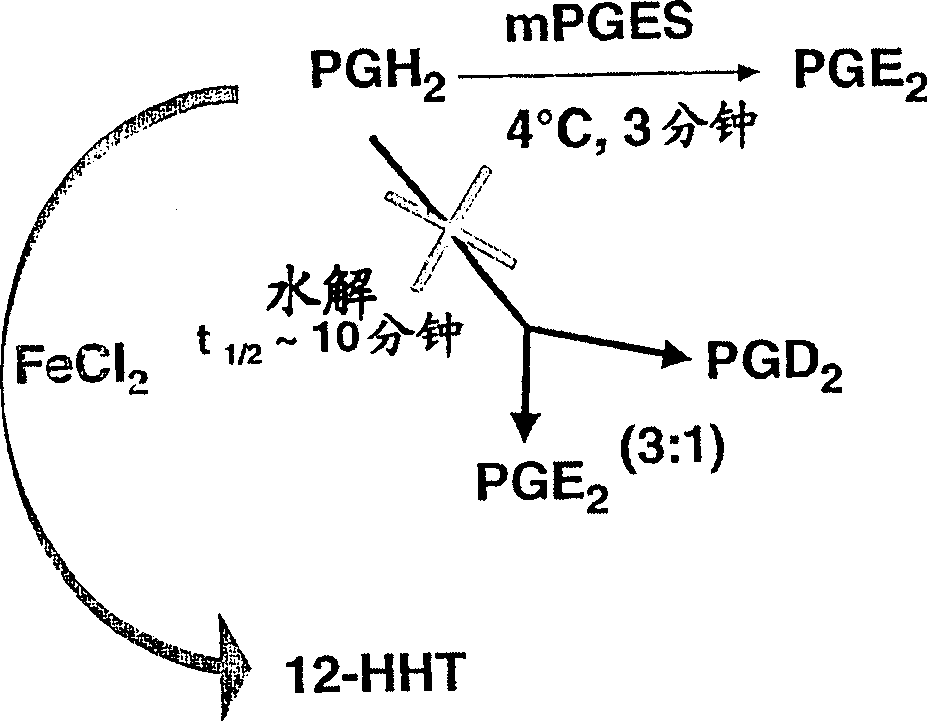
[0084] Prostaglandin E 2 (PGE 2 ) is a major mediator involved in inflammation and pain. Microsomal prostaglandin E synthase (mPGES) catalyzes PGH in the presence of glutathione 2 to PGE 2 transformation. It induces the expression of mPGES in many inflammatory diseases. Accordingly, compounds that reduce or inhibit the activity of mPGES would be useful agents for the treatment of inflammation, pain, fever, or combinations of these conditions, to name only a few diseases or disorders.
[0085] Inducible microsomal PGE has been developed 2 synthase to measure PGH 2 to PGE 2 Transformation test method. The test method is designed according to the principle of fluorescence polarization. PGH 2 , glutathione, and the compound or reagent being evaluated. After a short incubation (at least 30 seconds), add FeCl-containing 2 and a stop solution of citric acid to quench any r...
Embodiment II
[0107] Fluorescence polarization analysis of hematopoietic prostaglandin D synthase (hPGDS)
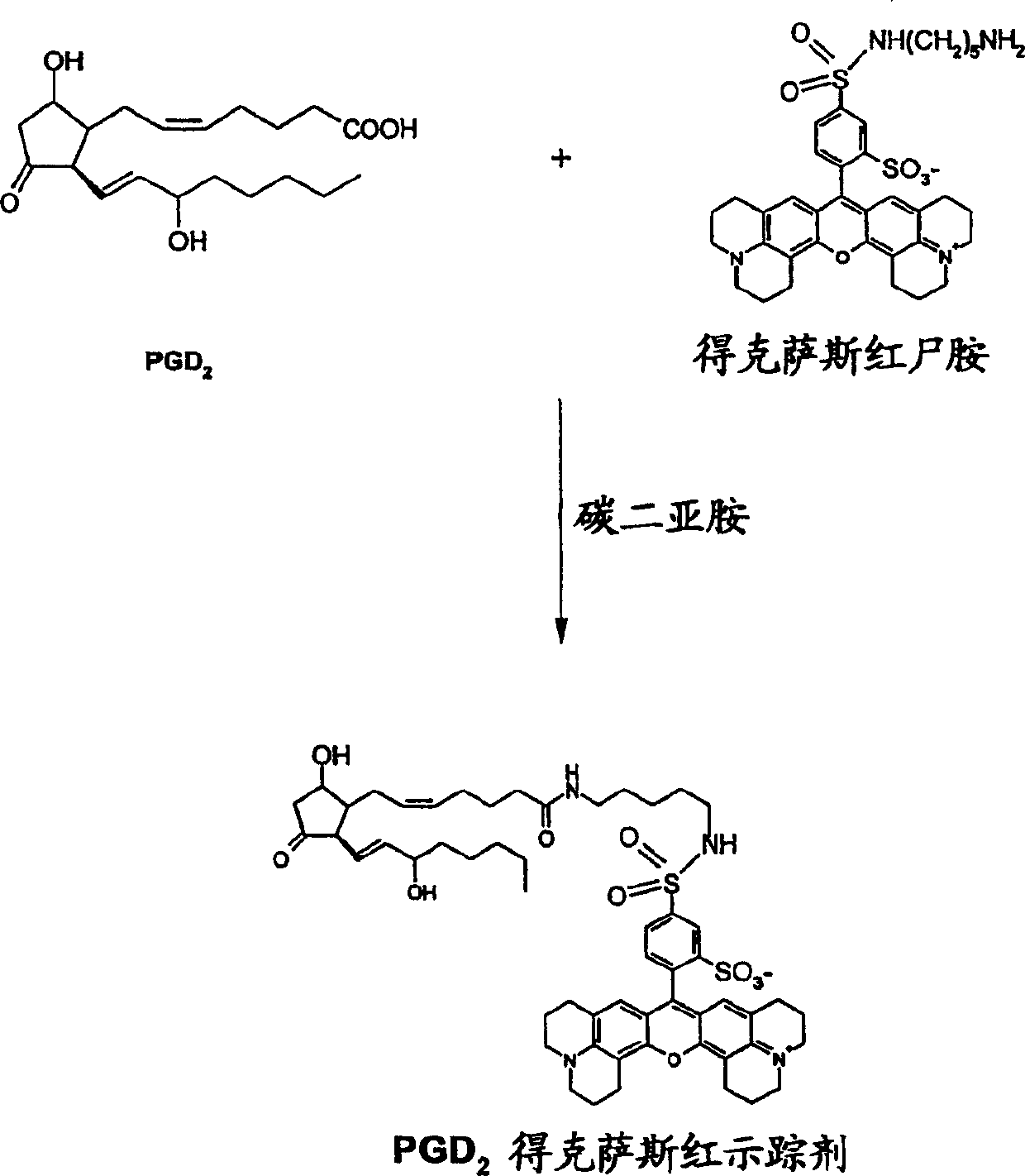
[0108] Antigenic stimulation will increase PGD in airway allergic diseases 2 generation. PGH is produced by hematopoietic prostaglandin D synthase (hPGDS) 2 Convert to PGD 2 PGD 2 , binds to both the D-type prostaglandin receptor (DP) and the chemokine receptor for Th2 cells (CRTH2), and increases bronchoconstriction, vasodilation, and dilatation of the nasal mucosa. The resulting bronchial hyperactivity, nasal obstruction, and infiltration of eosinophils and Th2 cells lead to allergic reactions. Therefore, compounds or agents that reduce or inhibit the activity of hPGDS can readily be used as therapeutic agents.
[0109] Fluorescence polarization (FP) assays (Figures 3 and 4), which measure hPGDS activity, were also investigated. The assay is designed based on the principle of fluorescence polarization. Combine hPGDS with PGH 2 , glutathione, and the compound or reagent bei...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com