Sapogenin derivatives, their synthesis and use and method based upon the use
A saponin and compound technology, applied in the field of sapogenin and its derivatives, can solve the problem of poor saponin effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0168] Cell-based analysis
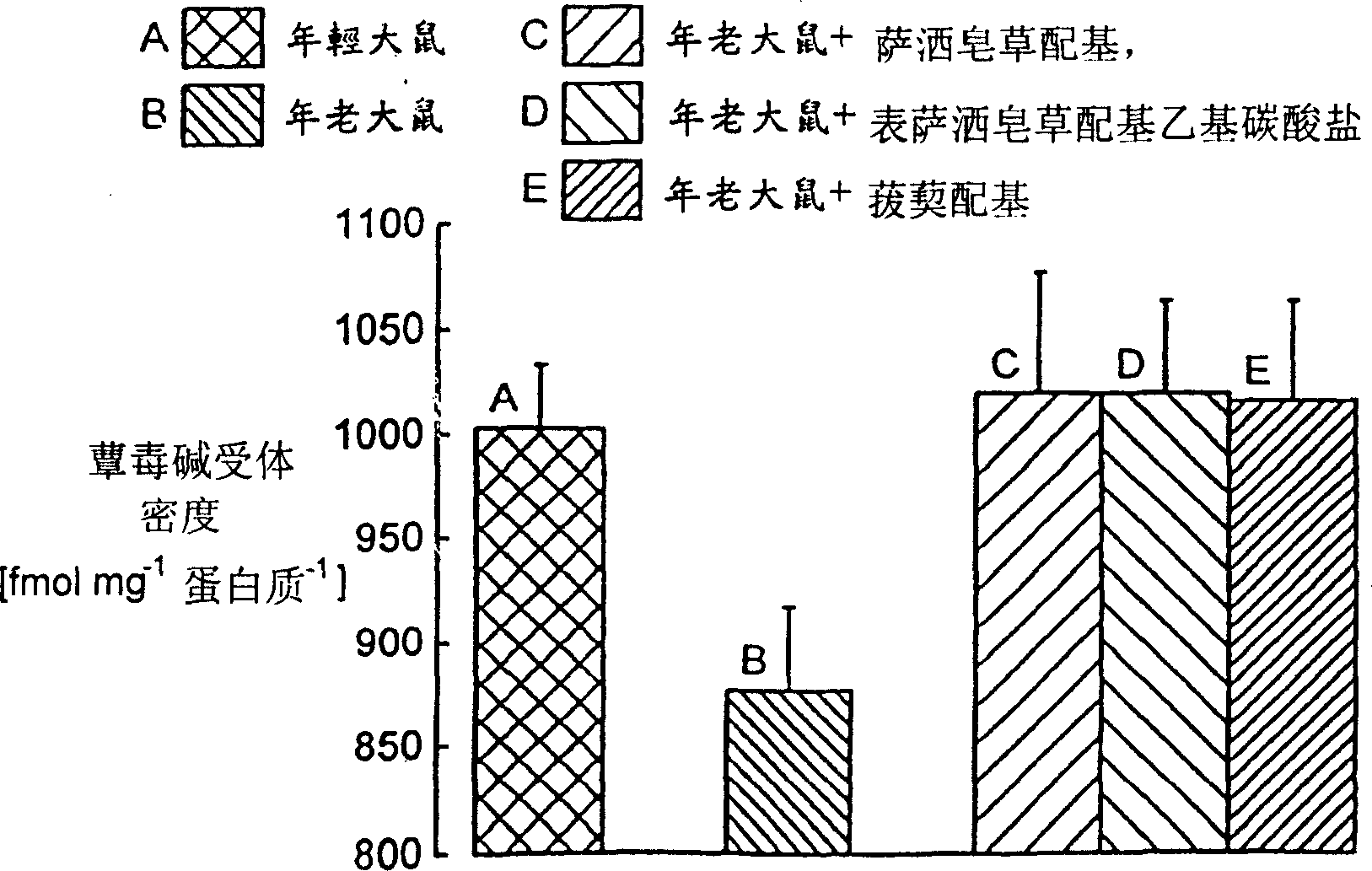
[0169] For episarsaporin formate ethyl acid, sarsasapogenin formate ethyl acid, episarsasogenin formate ethyl acid, episarsasogenin succinic acid, episarsasogenin acetic acid And the effect of sarsasapogenin on the expression of m receptor in CHO cells transfected by m receptor was observed. The number of receptors is used [ 3 H] NMS binding and subtracting non-specific binding analysis. The compound is dissolved in dimethyl sulfoxide (DMSO), and the control group is DMSO.
[0170] method:
[0171] On the day before the experiment, Chinese hamster ovary (CHO) cells with high muscarinic receptor expression (approximately 2.2 pmol per mg protein) were cultured in 24-well culture dishes. The culture medium was replaced with a culture medium containing a carrier (DMSO) or test compound. After 2 / 3 days, the culture medium was replaced once, and the cells were allowed to stand for another 2 / 3 days. The cell line was placed in a saturated concentration of N-m...
Embodiment 2
[0178] Alzheimer's disease pattern
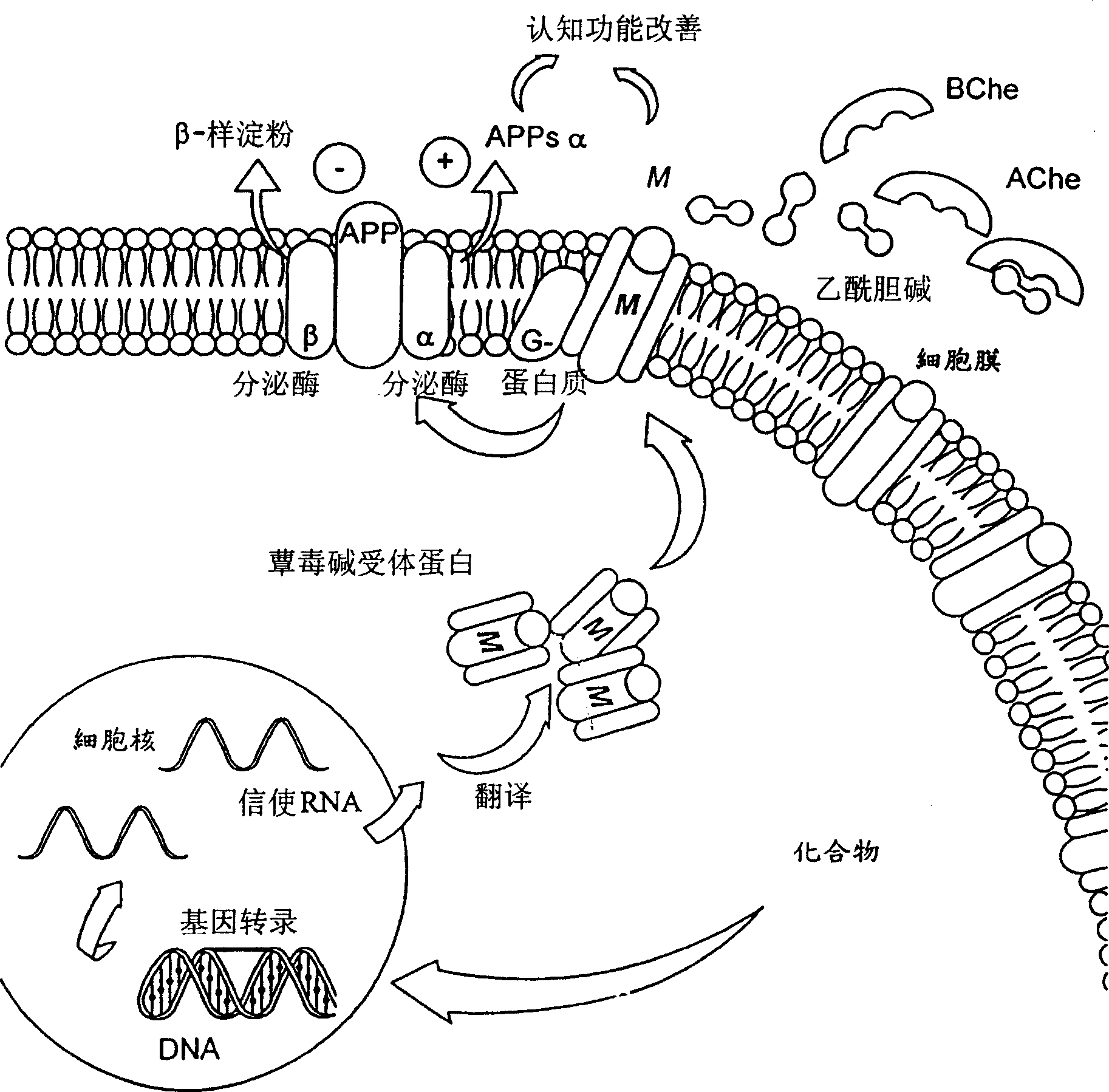
[0179] In a living Alzheimer's disease model, amyloid beta and ibotenic acid are injected into the brain of rats, causing loss of receptors in the brain and cognitive impairment. Previous studies have shown that local injection of starch-like β into the nucleus vasalis of the rat brain leads to hypocholinergic function and behavioral impairment for up to 2 months after surgery (Giovannelli et al., 1995: Neuroscience, 66, 781-792). In addition, at the same time, amyloid β and a small amount of ibotenic acid were transferred to the hippocampus of rats in a benign and synergistic manner to produce nerve loss, accompanied by the penetration of ganglion cells to the surrounding and distant parts of the injection site (Morimoto et al., 1998 : Neuroscience, 84, 479-487).
[0180] method:
[0181] Our study used Morimoto's method (Morimoto et al., 1998: Neuroscience, 84, 479-487), with some modifications (instead of bilateral injection with unilateral ...
Embodiment 3
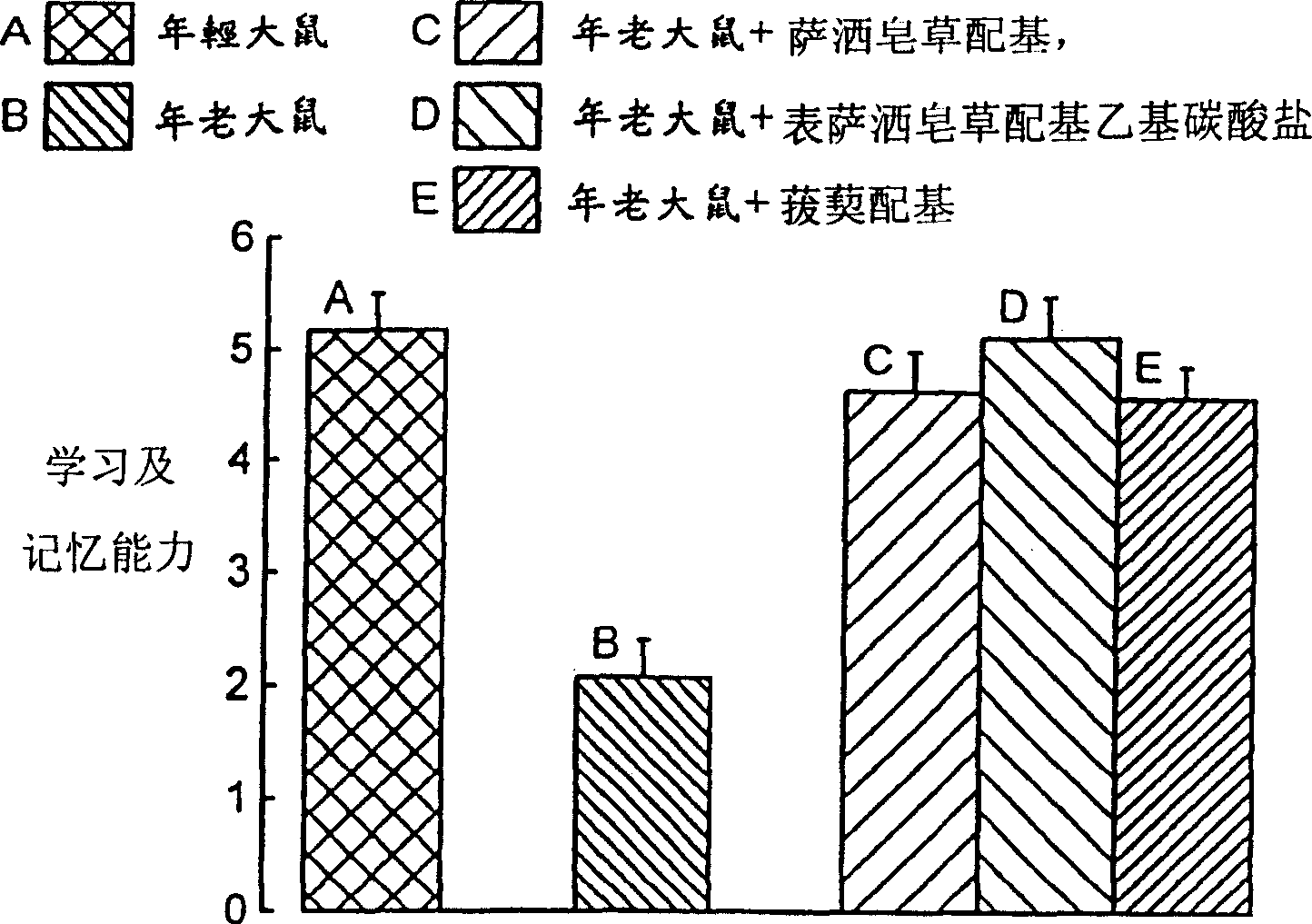
[0204] Learning and memory test
[0205] The old Sprague-Dawley rats were randomly divided into 4 groups, a control group, which seemed to receive sarsasapogenin, episarasapogenin ethyl carbonate, or smilaxin (18 mg per day). / kg, n=10) 3 groups up to 3 months. A control group (n=14) composed of young rats that did not receive any treatment is also included in this example. The daily dose of the drug is mixed with a minimum amount of food and given to individual rats every morning.
[0206] Y-maze is used for learning and memory test. There is an array of copper rods on the floor of each branch of the Y-maze, which can be supplied with currents of different voltages when needed. Each branch is 45cm long and has a 15W bulb at the end, which can be lit when needed. After using the drug for 3 months, each rat received the following training for 7 consecutive days. During each training period, the rat was placed in a branch of the Y-maze. After resting for 2 minutes, an electric curre...
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap