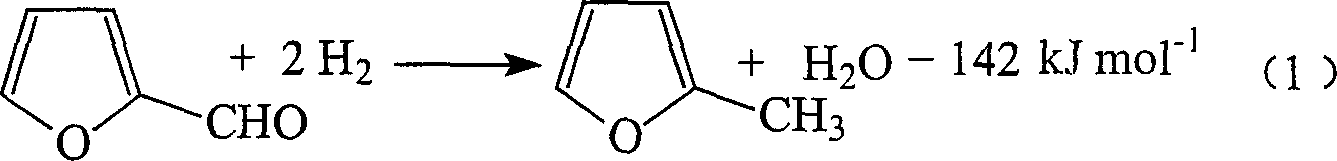
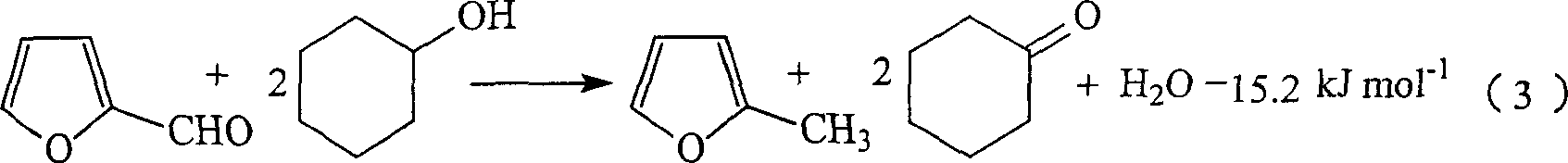
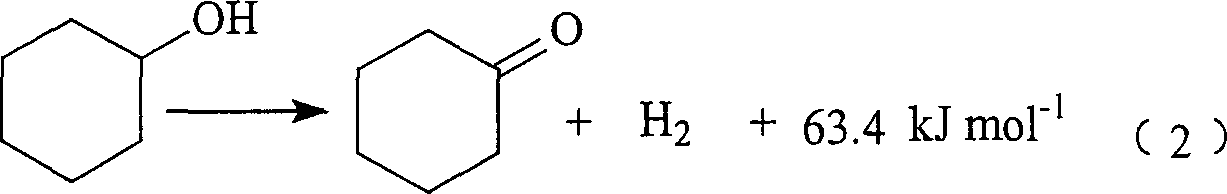Preparation of 2-methylfuran and cyclohexanone by couple method
A technology of methyl furan and cyclohexanone, which is applied in the field of preparation of 2-methylfuran and cyclohexanone, can solve the problems of waste, high liquid air cannot be fully utilized, and the conversion rate of cyclohexanone is not high, so as to achieve less energy , The effect of saving hydrogen production equipment
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0061] In the gas-phase fixed-bed reactor (the installed catalyst composition and activation reduction and reactor structure are the same as Comparative Example 1), respectively vaporized cyclohexanol and furfural enter the reactor after mixing with circulating hydrogen in a 4.3:1 molar ratio. At normal pressure, the total space velocity of liquid is 1.0hr -1 1. Under the condition that the molar ratio of circulating hydrogen to the mixture is 5:1, when the temperature is 250°C, the conversion rate of cyclohexanol is 48.6%, the conversion rate of furfural is 99.7%, the selectivity of cyclohexanone is 95.8%, and the 2-methyl The selectivity of methyl furan is 90.7%; when the reaction temperature is 270°C, the conversion rate of cyclohexanol is 62.1%, the conversion rate of furfural is 99.9%, the selectivity of cyclohexanone is 93.4%, and the selectivity of 2-methylfuran is 86.4%; normal operation After the reaction, no external supply of hydrogen is required.
Embodiment 2
[0063] In the gas-phase fixed-bed reactor (the installed catalyst composition and activation reduction and reactor structure are the same as comparative example 1), under normal pressure, 260 ℃ of reaction temperature, respectively vaporized cyclohexanol, furfural are by 4: 1 molar ratio, circulate The molar ratio of hydrogen to mixed raw materials is 10:1, and the total liquid space velocity is about 0.7hr -1 Conditions, the conversion rate of cyclohexanol is 53.6%, the conversion rate of furfural is 99.9%, the selectivity of cyclohexanone is 94.5%, and the selectivity of 2-methylfuran is 88.6%.
Embodiment 3
[0065] In the gas-phase fixed-bed reactor (the installed catalyst composition and activation reduction and reactor structure are the same as comparative example 1), reaction pressure normal pressure, reaction temperature 270 ℃, respectively vaporized cyclohexanol, furfural by 3.6: 1 molar ratio, The molar ratio of circulating hydrogen to mixed raw materials is 25:1, and the total liquid space velocity is about 0.6hr -1 Conditions, the conversion rate of cyclohexanol is 59.0%, the conversion rate of furfural is 99.6%, the selectivity of cyclohexanone is 94.8%, and the selectivity of 2-methylfuran is 87.8%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com