3-amido-4-alkylamino lauseto neu preparation method
A technology of alkylaminophenylmethyl sulfone and nitrophenylmethyl sulfone, which is applied in the field of preparation of 3-amino-4-alkylaminophenylmethyl sulfone, which can solve the problems of raw material sources, low reduction yield, environmental pollution, etc. problems, to achieve the effect of mild conditions, high purity, and less waste
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
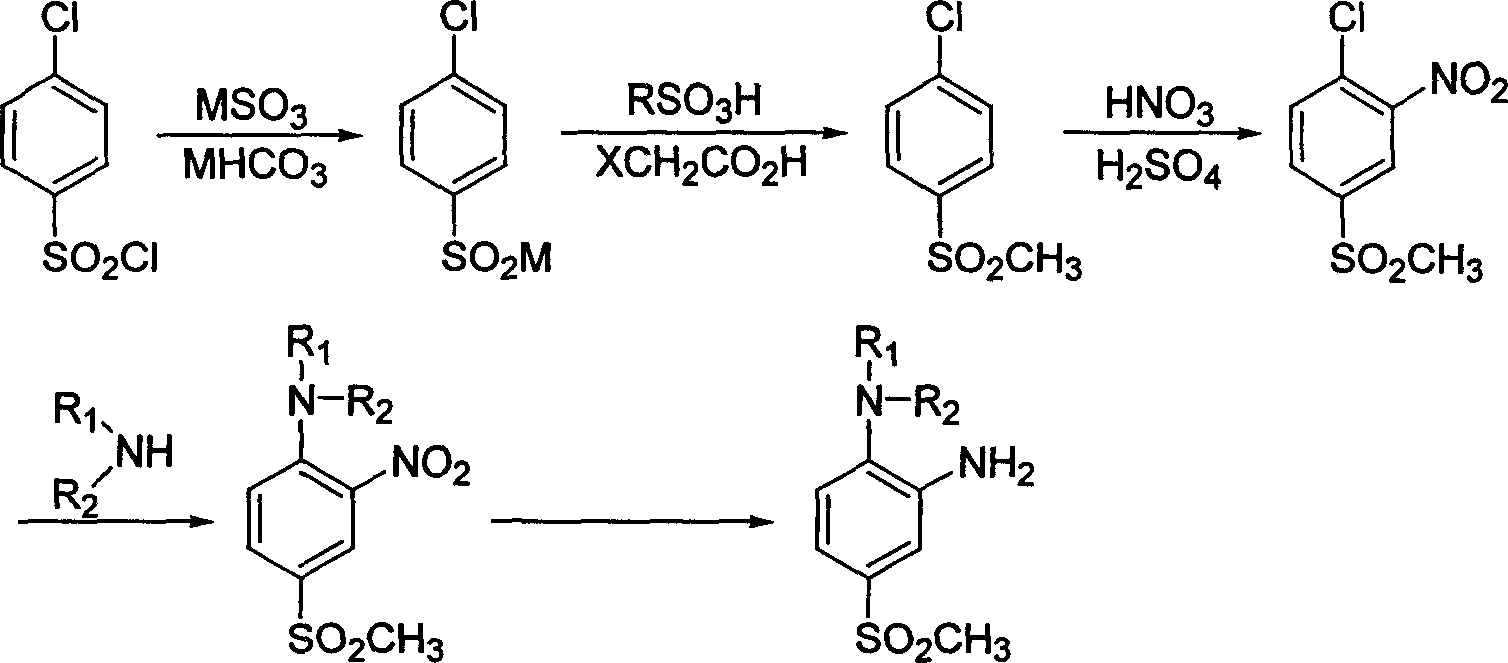
[0016] The first step of synthesis of sodium p-chlorobenzenesulfinate
[0017] Add 144g (1.14mol) of anhydrous sodium sulfite, 820ml of water, 240g (2.86mol) of anhydrous sodium bicarbonate into a 2000ml four-neck flask, heat the oil bath to 70°C, add 233.6g (1.11mol) of p-chlorobenzenesulfonyl chloride, Keep warm at 70-80°C, stir for 2.5 hours, cool down to room temperature, and use it directly for the next reaction.
[0018] Synthesis of p-chlorophenylmethyl sulfone in the second step
[0019] Add 127.5 g (1.3 mol) of chloroacetic acid and 18 g of p-toluenesulfonic acid to the reaction liquid in the previous step, heat up the oil bath to 105° C., and reflux for 8 hours until no bubbles bubble out in the reaction liquid. Cool in a water bath, filter with suction, wash with water three times, and dry to obtain 193.2 g of p-chlorophenylmethyl sulfone as a white granular solid, mp: 95-96°C, yield 91.6%.
[0020] The third step of synthesis of 4-chloro-3-nitrophenyl methyl sulf...
Embodiment 2
[0027] The first step of synthesis of sodium p-chlorobenzenesulfinate
[0028] Add 181.7g (1.15mol) of anhydrous potassium sulfite, 820ml of water, 300g (3.0mol) of anhydrous potassium bicarbonate into a 2000ml four-neck flask, heat up the oil bath to 70°C, add 233.6g (1.11mol) of p-chlorobenzenesulfonyl chloride mol), kept at 70-80°C, stirred and reacted for 2 hours, cooled down to room temperature, and used directly for the next reaction.
[0029] Synthesis of p-chlorophenylmethyl sulfone in the second step
[0030] 154.3 g (1.11 mol) of bromoacetic acid and 18 g of p-toluenesulfonic acid were added to the reaction solution in the previous step, and the oil bath was heated to 90° C., and the rest was the same as in the second step of Example 1 to obtain white granular solid p-chlorophenylsulfone.
[0031] The third step of synthesis of 4-chloro-3-nitrophenyl methyl sulfone
[0032] Slowly add 40.5ml (74.5g) of concentrated sulfuric acid to 192.2ml (296g) of fuming nitric a...
Embodiment 3
[0038] The first step of synthesis of sodium p-chlorobenzenesulfinate
[0039] Add 153.8g (1.22mol) of anhydrous sodium sulfite, 850ml of water, 186.5g (2.22mol) of anhydrous sodium bicarbonate into a 2000ml four-neck flask, heat the oil bath to 70°C, add 233.6g (1.11mol) of p-chlorobenzenesulfonyl chloride ), kept at 85-90°C, stirred and reacted for 3 hours, cooled down to room temperature, and used directly for the next reaction.
[0040] Synthesis of p-chlorophenylmethyl sulfone in the second step
[0041] Add 131.1 g (1.39 mol) of chloroacetic acid and 20 g of methanesulfonic acid to the reaction solution in the previous step, and heat up the oil bath to reflux. The rest is the same as the second step of Example 1 to obtain white granular solid p-chlorophenylmethyl sulfone.
[0042] The third step of synthesis of 4-chloro-3-nitrophenyl methyl sulfone
[0043]Slowly add 64.5ml (118.7g) of concentrated sulfuric acid to 163.4ml (251.6g) of fuming nitric acid to prepare a mi...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com