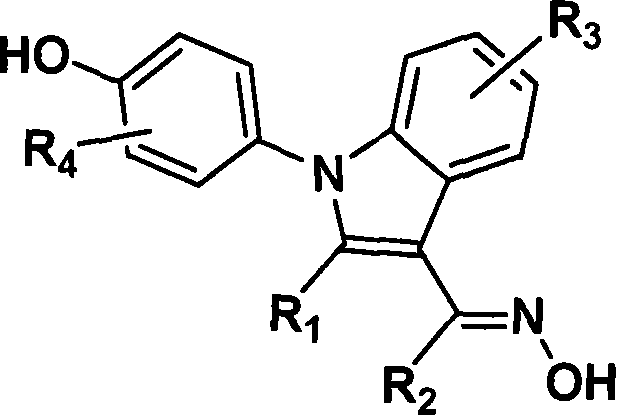
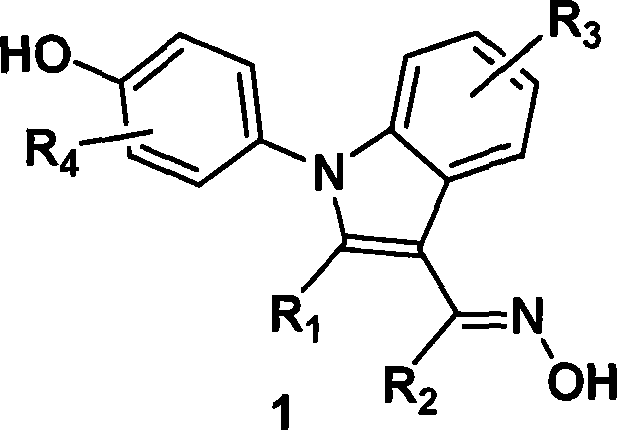
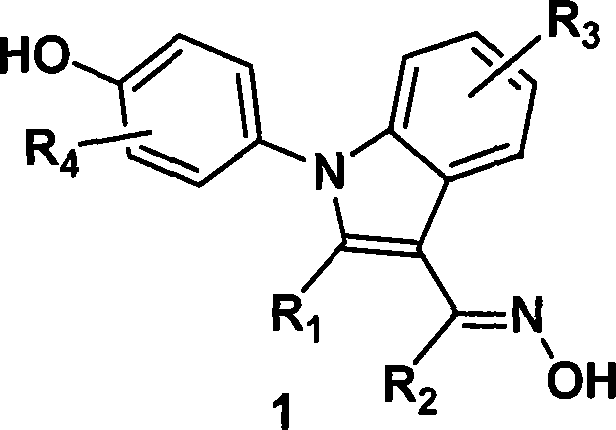
(4-hydroxyphenyl)-1h-indole-3-carbaldehyde oxime derivative as estrogenic agents
A technology of hydroxyphenyl and formaldehyde oxime, applied in the field of -1H-indole-3-formaldehyde oxime derivatives
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1a
[0059] 1-(4-Benzyloxyphenyl)-2-methyl-1H-indole
[0060] To a mixture of 2-methylindole (3.28 g, 25 mmol), 4-benzyloxyiodobenzene (7.76 g, 25 mmol), potassium carbonate (2.65 g, 25 mmol), and N-methylpyrrolidone (250 mL) was added Cuprous (I) bromide (0.5 g, 3.5 mmol). The stirred solution was heated to 180°C for 18 hours. After the reaction mixture was cooled, it was poured onto ice and extracted with ethyl acetate. After washing with water and brine, the organic layer was washed with anhydrous MgSO 4 Drying and removal of solvent gave a dark liquid. Purification by silica gel chromatography (2% ethyl acetate-hexanes) afforded the title compound (30%) as a white solid: mp 95-96°C; 1 H NMR (DMSO-d 6 ): δ2.24(3H, s), 5.19(2H, s), 6.38(1H, s), 6.93-7.04(3H, m), 7.20(2H, d, J=1.9Hz), 7.23(2H, d, J=3.1Hz), 7.32-7.53 (8H, m); MS m / z (M+H) + 314:
[0061] to C 22 h 19 NO0.1H 2 Analysis of O:
[0062] Calculated: C, 83.83; H, 6.14; N, 4.44.
[0063] Found: C, 83.84; H, 6...
Embodiment 1b
[0065] 5-fluoro-1-(4-benzyloxyphenyl)-2-methyl-1H-indole
[0066] 5-Fluoro-2-methylindole (5.0 g, 33.5 mmol) was reacted with 4-benzyloxyiodobenzene (10.40 g, 33.5 mmol) according to the procedure used in the previous example 1a to give a white solid (23%) : mp 94-95°C; 1 H NMR (DMSO-d 6 ): δ2.23(3H, s), 5.19(2H, s), 6.39(1H, s), 6.84-6.94(2H, m), 7.19-7.29(3H, m), 7.34-7.44(5H, m ), 7.51 (2H, d, J=7.1Hz); MS m / z (M+H) + 332.
[0067] to C 22 h 18 Analysis of NOF:
[0068] Calculated: C, 79.74; H, 5.47; N, 4.23.
[0069] Found: C, 79.54; H, 5.54; N, 4.21.
Embodiment 1c
[0071] 5-Chloro-1-(4-methoxymethyloxyphenyl)-2-methyl-1H-indole
[0072] 5-Chloro-2-methylindole (4.14g, 25mmol) was reacted with 4-methoxymethyloxyiodobenzene (6.20g, 25mmol) according to the steps adopted in previous Example 1a to obtain a colorless liquid ( 19%): 1 H NMR (DMSO-d 6 ): δ2.25(3H, s), 3.44(2H, s), 5.28(2H, s), 6.40(1H, s), 6.96(1H, d, J=8.8Hz), 7.02(1H, dd, J=2.0Hz, J=8.8Hz), 7.22 (2H, d, J=8.8Hz), 7.36 (2H, d, J=8.8Hz), 7.55 (1H, d, J=2.0Hz); MS m / z(M+H) + 302 / 304 (1Cl).
[0073] to C 17 h 16 ClNO 2 Analysis of:
[0074] Calculated: C, 67.66; H, 5.34; N, 4.64.
[0075] Found: C, 67.49; H, 5.17; N, 4.51.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com