Immuno colloidal gold test paper strip for detecting sulfa drug residue
A technology of sulfonamide drugs and test strips, which is applied in the field of immunochemical rapid detection of veterinary drug residues, can solve the problems of complex operation process, high requirements for instrument maintenance, and high detection costs, and achieve low storage temperature requirements, multi-function and The effect of good specificity and low detection cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0040] Embodiment 1 (preparation embodiment)
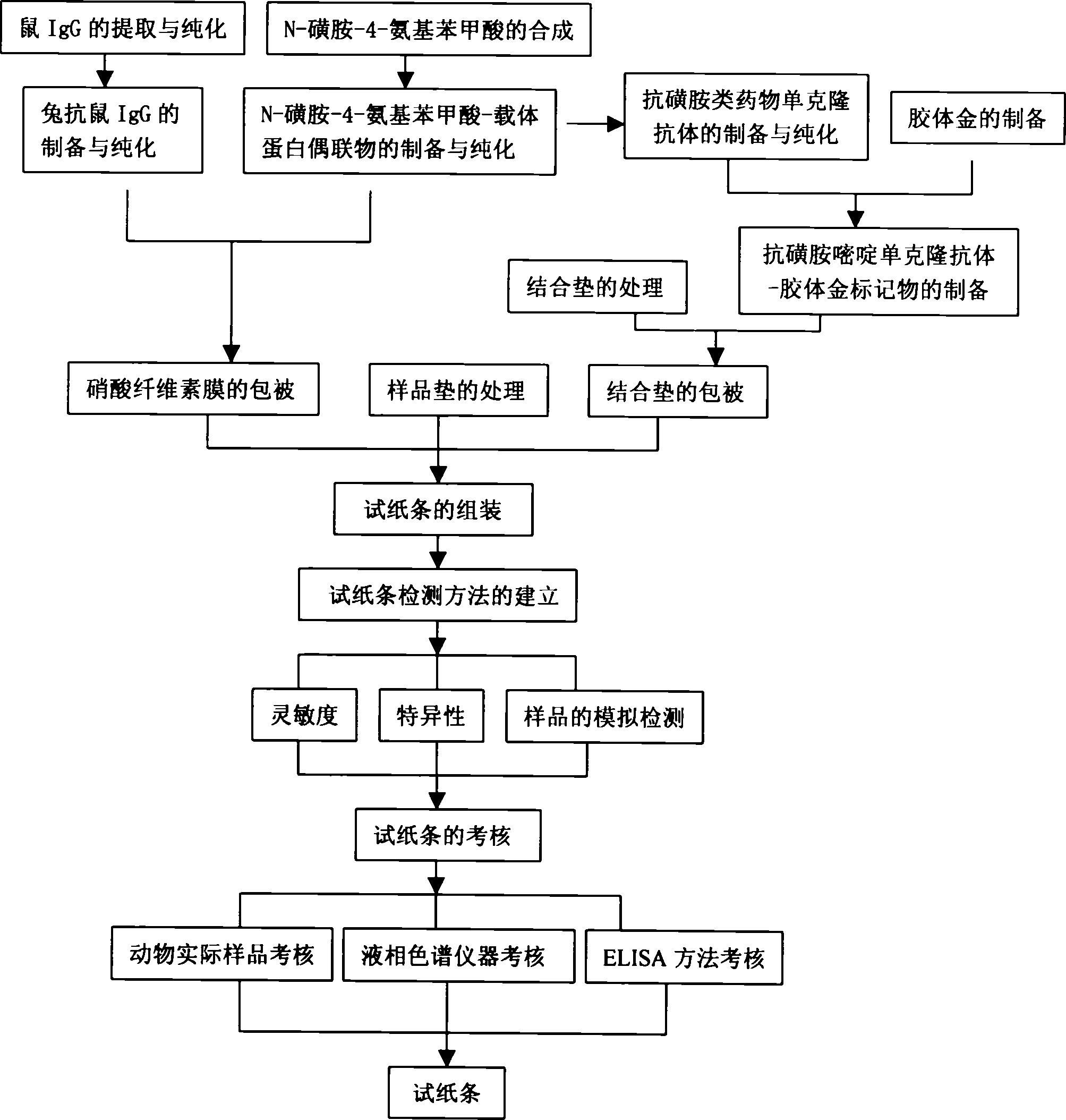
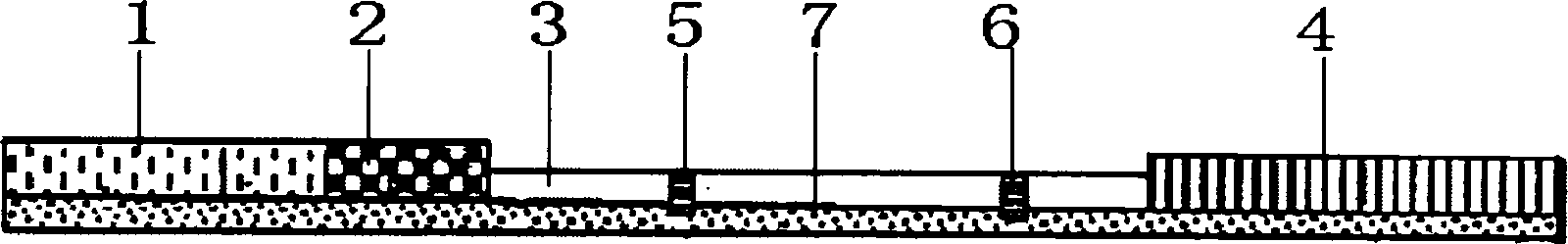
[0041] Preparation method of immunocolloidal gold test strip for detecting sulfa drugs
[0042] 1. Synthesis of the small molecular hapten N-sulfa-4-aminobenzoic acid (SDL), which contains p-aminobenzenesulfonamide, the common core structure of sulfa drugs. The specific steps are:
[0043] (1) Accurately weigh 3.2 g of methyl p-aminobenzoate, dissolve it in 20 mL of anhydrous pyridine, and pour it into a 100 mL two-neck round bottom flask.
[0044] (2) While stirring, slowly add 6.0 g of vacuum-dried p-acetamidobenzenesulfonyl chloride into the round bottom flask, and after the addition, reflux on the oil bath to make the solution turn red.
[0045] (3) After refluxing for a few minutes, the reaction solution turned into a very viscous red substance, and after a few minutes the reaction solution turned into a brown liquid, and continued to reflux for 15 minutes, and the reaction solution turned into a brownish-yellow liquid. Af...
Embodiment 2
[0079] Embodiment 2 (application embodiment)
[0080] The use method of the immunocolloidal gold test strip for detecting sulfa drugs of the present invention
[0081] 1. Sample pretreatment
[0082] (1) Pretreatment of tissue samples
[0083] Homogenize the tissue sample to be tested, take 2 g of the tissue sample to be tested, add 4 mL of ethyl acetate, shake up and down for 5 min, centrifuge at 2000 g at room temperature for 5 min, take 300 μL of the upper layer liquid and evaporate it to dryness in a water bath at 80 ° C or dry it under nitrogen flow, and use 150 μL of pH7.4 Phosphate buffer (recipe: NaCl 8g, KCl 0.2g, NaCl 2 HPO 4 12H 2 O 2.9g, KH 2 PO 4 0.2 g, dilute to 1000 mL with distilled water) to dissolve the residue.
[0084] (2) Pretreatment of milk samples
[0085] After the milk sample was degreased by centrifugation at 3500rpm for 10min, absorb 2mL of the middle layer of milk and add 4mL of ethyl acetate to shake up and down for 5min, centrifuge at 20...
Embodiment 3
[0092] Embodiment 3 (application embodiment)
[0093] Application of the immunocolloidal gold test strip for detecting sulfa drugs of the present invention
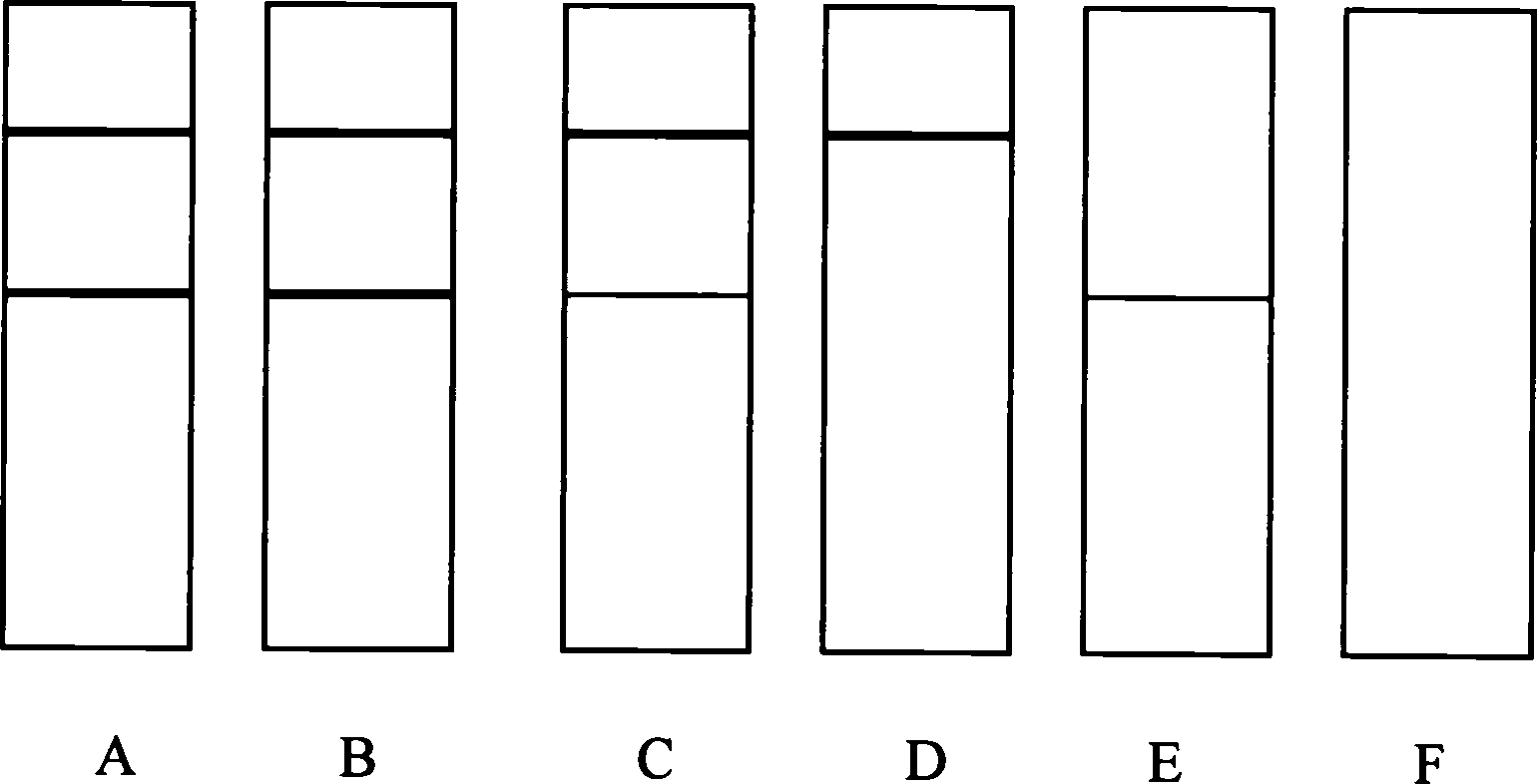
[0094] 1. Specificity test
[0095] According to the method described in Example 2, it is 10, 20, 40, 80, 100 ng / mL sulfamethoxine and the negative standard substance that are respectively detected with the detection test strip of the present invention, and are measured with the Biodot TSR3000 strip reading system. The detection line absorbance value (G / Peak-ROD value), taking the concentration of sulfamethoxine as the X axis, and the detection line absorbance value (G / Peak-ROD value) as the Y axis draw a standard curve, and calculate the sulfamethoxine according to the standard curve The 50% inhibitory concentration of oxypyrimidine was 18.3ng / mL. Similarly, the 50% inhibitory concentration of sulfamethoxine, sulfadimethoxine, sulfadiazine, sulfaquinoxaline, sulfamethazine, sulfamethoxazole, and trimethoprim was calcul...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Sensitivity | aaaaa | aaaaa |
| Sensitivity | aaaaa | aaaaa |
| Sensitivity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com