Dispersing tablets for clearing heat,purging fire and detoxifying
A detoxification function, heat-clearing and fire-clearing technology, applied to medical preparations containing active ingredients, plant/algae/fungus/moss ingredients, plant raw materials, etc., can solve the problem of high proportion content, difficult industrialization, and disintegration of traditional Chinese medicine dispersible tablets It is difficult to control the speed and other problems, so as to achieve the effect of improving disintegration and large room for maneuver
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0054] The traditional Chinese medicine raw materials of Yiqing Dispersible Tablets: Coptis 165 grams, Rhubarb 500 grams, Scutellaria baicalensis 250 grams.
[0055] The weight percentage of traditional Chinese medicine extract and auxiliary materials of Yiqing dispersible tablet: fine powder of Yiqing extract 24%, low-substituted hydroxypropyl cellulose 22%, sodium carboxymethyl starch 18%, microcrystalline cellulose 10%, lactose 20%, Polyvinylpyrrolidone 3%, Magnesium Stearate 3%.
[0056] Yiqing Dispersible Tablets are obtained according to the following specific steps: Coptidis rhizome, rhubarb, and scutellaria baicalensis, and the above three flavors are decocted twice with water, the first time is 1.5 hours, the second time is 1 hour, the decoctions are combined, filtered, and the filtrate is concentrated under reduced pressure to The relative density is about 1.25 (70°C), spray-dried and crushed to obtain a fine powder of Yiqing extract.
[0057] First pass the above-m...
Embodiment 2
[0059] Yiqing extract powder 30% by weight, sodium carboxymethylcellulose 20%, microcrystalline cellulose 12%, cross-linked polyvinylpyrrolidone 16%, polyvinylpyrrolidone 5%, lactose 10%, polyvinylpyrrolidone 4%, micronized silica gel 1%, magnesium lauryl sulfate 2%. All the other are with embodiment 1.
Embodiment 3
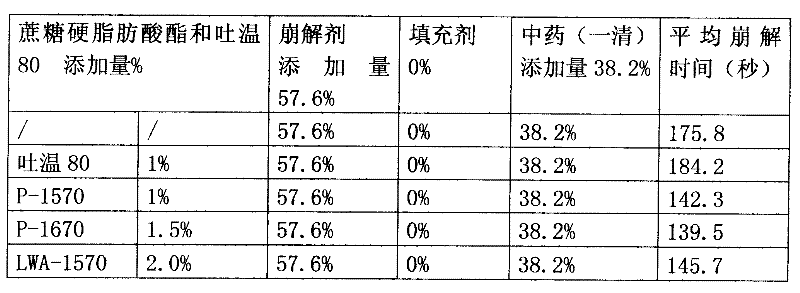
[0061] Yiqing extract powder 36% by weight, sodium carboxymethyl starch 20%, croscarmellose sodium 15%, crospovidone 6%, low-substituted hydroxypropyl cellulose 5%, Mannitol 7%, polyvinylpyrrolidone 7%, micronized silica gel 2%, magnesium stearate 1%, HLB=15-16 sucrose fatty acid ester 1%. Sucrose fatty acid ester is P-1570 or P-1670 or LWA-1570, all the other are the same as embodiment 1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com