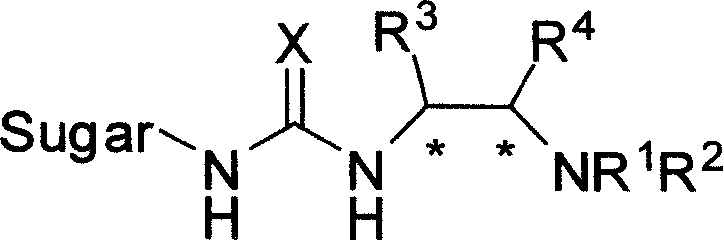
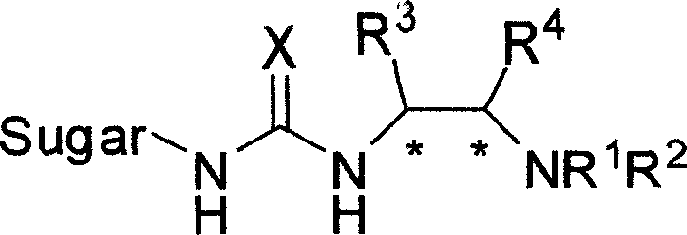
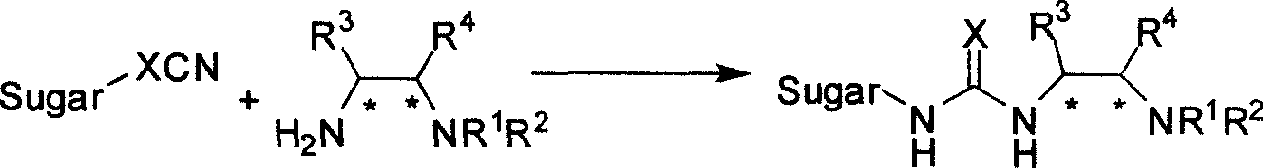Bifunctional chiral sacchariferous semicarbazide and thiocarbamide catalyst and its prepn process and application in asymmetric reaction
A chiral catalyst and dual-functional technology, applied in chemical instruments and methods, preparation of organic compounds, physical/chemical process catalysts, etc., can solve problems such as inability to obtain ideal results for substrates, and achieve high stereoselective effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0031] Example 1: Preparation of catalyst: N-(1R, 2R)-(2-aminocyclohexyl)-N'-(2',3',4',6'-tetra-O-acetyl-β-D- Glucopyranosyl-1'-)thiourea
[0032]
[0033] At room temperature, 1.14 g (3 mmol) of 2,3,4,6-tetra-O-acetyl-β-D-glucopyranosyl isothiocyanate was added to dissolve 0.41 g (3.6 mmol) (1R, 2R)-cyclohexanediamine in dichloromethane (20 mL), and then the mixture was reacted at the same temperature for 6 hours. After the solvent was distilled off, the mixture was subjected to silica gel column chromatography using ethyl acetate / triethylamine (volume ratio 100 / 1) as the eluent to obtain the crude product. At 0-5°C, dissolve it with as little dichloromethane as possible. After slowly adding petroleum ether (60-90°C), the required chiral thiourea catalyst is precipitated with a yield of 61%, [α] D 20 +43.1°(c 1.0, CHCl 3 ), 1 H NMR(500MHz, CDCl 3 )δ5.82(m, 1H), 5.21(d, J=80Hz, 4H), 4.30-3.83(m, 4H), 3.48(m, 1H), 2.00-2.09(m, 14H).1.72(m, 3H), 1.26 (m, 5H), 0.93 (m, 1H); IR (KB...
example 2
[0034] Example 2: Preparation of catalyst: N-(1R, 2R)-(2-aminocyclohexyl)-N'-(2', 3', 4', 6'-tetra-O-acetyl-β-D- Glucopyranosyl-1'-)thiourea
[0035]
[0036] At room temperature, 1.14 g (3mmol) 2,3,4,6-tetra-O-acetyl-β-D-glucopyranosylamine was added to dissolve 0.41 g (3.6mmol) (1R, 2R)-1 -Amino-2-iso(thio)hydrocyclohexane in dichloromethane (20 mL), and then the mixture was reacted at 40°C for 2 hours. After the solvent was distilled off, the mixture was subjected to silica gel column chromatography using ethyl acetate / triethylamine (volume ratio 100 / 1) as the eluent to obtain the crude product. At 0-5°C, dissolve it with as little dichloromethane as possible. After slowly adding petroleum ether (60-90°C), the required chiral thiourea catalyst is precipitated with a yield of 52%, [α] D 20 +43.1°(c 1.0, CHCl 3 ),1 HNMR(500MHz, CDCl 3 )δ 5.82 (m, 1H), 5.21 (d, J = 80Hz, 4H), 4.30-3.83 (m, 4H), 3.48 (m, 1H), 2.00-2.09 (m, 14H), 1.72 (m, 3H), 1.26 (m, 5H), 0.93 (m, 1H); IR (KBr)...
example 3
[0037] Example 3: Preparation of catalyst: N-(1R, 2R)-(2-aminocyclohexyl)-N'-(2', 3', 4', 6'-tetra-O-pivaloyl-β-D -Glucopyranosyl-1'-)thiourea
[0038]
[0039] Synthesis of N-(1R,2R)-(2-aminocyclohexyl)-N'-(2',3',4',6'-tetra-O-pivaloyl-β-D by a method similar to Example 1 -Glucopyranosyl-1'-)thiourea, the yield is 33%, [α] D 20 +63.1°(c 1.0, CHCl 3 )
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap