Methods for enhancing the potency of the immune checkpoint inhibitors
a technology potent immune response, which is applied in the field can solve the problems of patients who cannot mount a potent immune response to fully eliminate cancer cells, significant proportion of responders experience tumor relapse, and high liver toxicity of patients with braf-mutated melanoma. achieve the effect of enhancing the potency of immune checkpoint inhibitors
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example
[0061]Material & Methods
[0062]Cell Culture
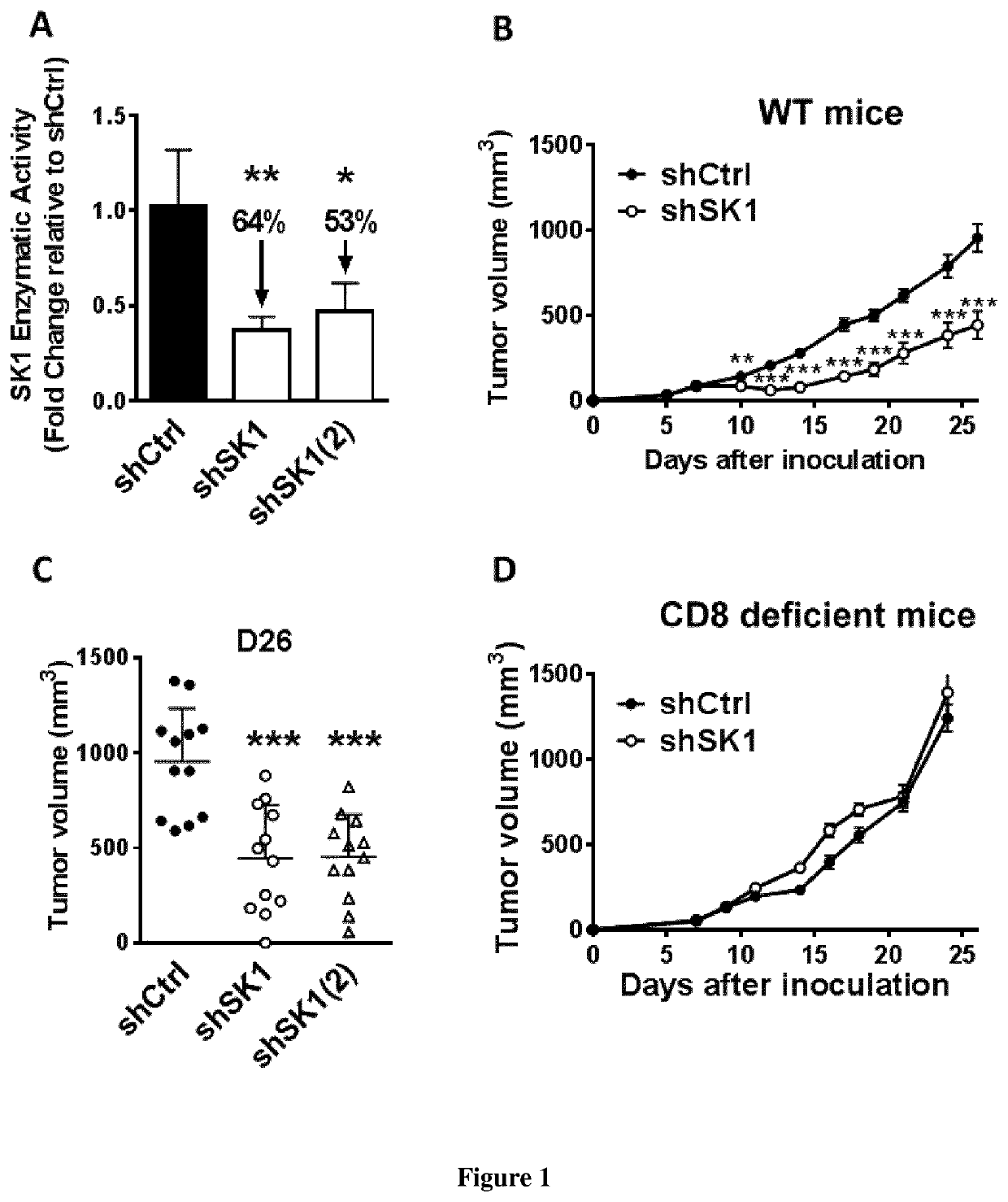
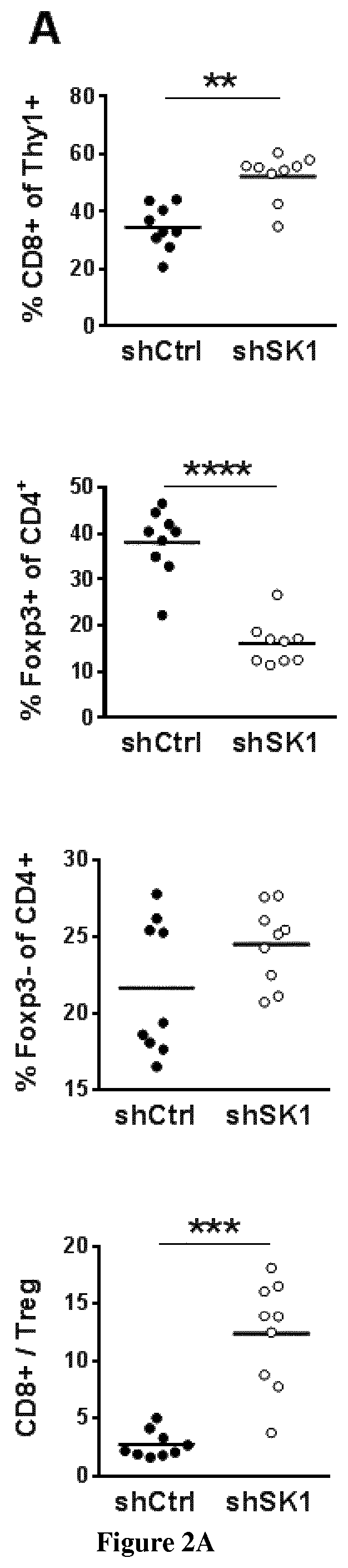
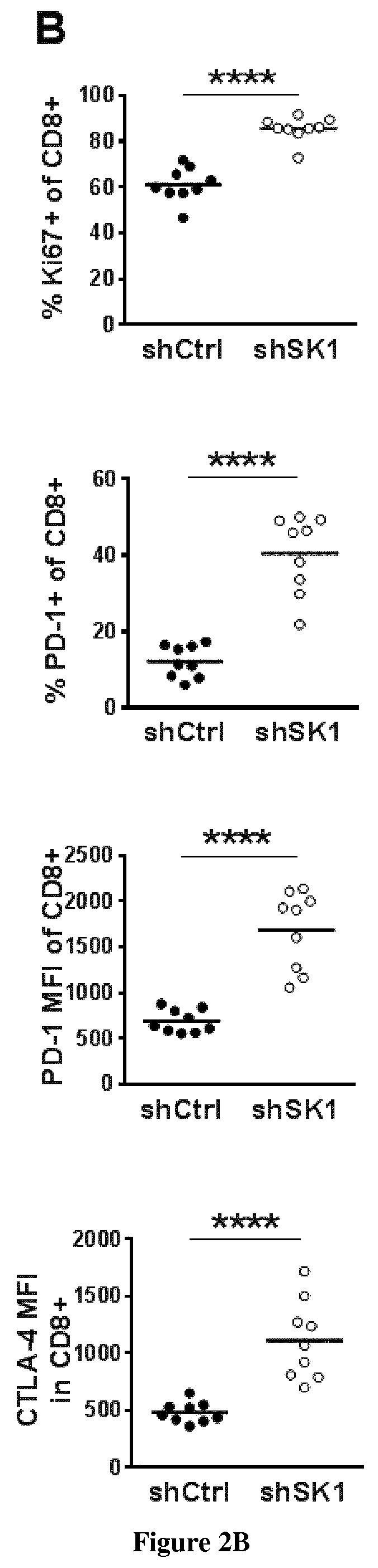
[0063]Yumm murine melanoma cells, which harbor BRAFV600E mutation, Pten and Cdkn2a deletion [1] were kindly provided by Dr. S. Tartare-Deckert (INSERM U1065 Nice, France). Yumm cells were grown as monolayers in OptiMEM media supplemented with 3% heat-inactivated fetal calf serum (FCS) in the presence of 5% CO2 in a humidified atmosphere at 37° C. To guarantee cell line authenticity, Yumm cell lines were used for a limited number of passages and routinely tested for the expression of melanocyte-lineage proteins such as MelanA / MART1. MC38 cells were kindly provided by Drs T. Chardes et A. Pélegrin (INSERM U1194, Montpellier, France) and were cultured in DMEM containing 10% FCS, 2 mM glutamine, 0.1 mM non essential amino acids, 1 mM sodium pyruvate and 10 mM Hepes.
[0064]Cell Transfection
[0065]Yumm cells were co-transfected, in a 1:10 ratio, with the pEGFP-N empty vector and a SK1 shRNA (shSK1 or shSK1(2)) plasmid (shRNA from Thermoscientific) o...
PUM
| Property | Measurement | Unit |
|---|---|---|
| plasma dehydrogenase | aaaaa | aaaaa |
| reduction of function | aaaaa | aaaaa |
| time | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com