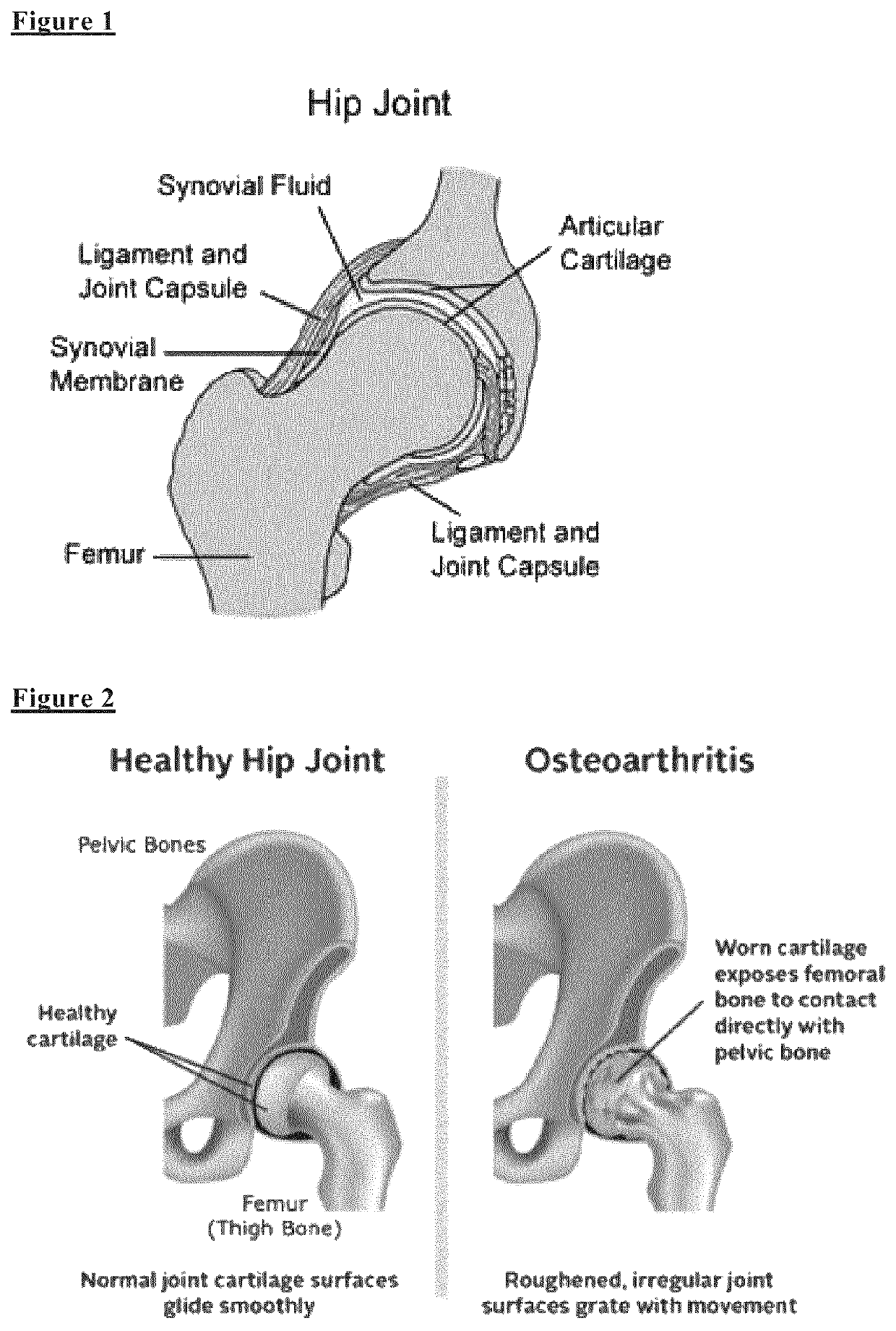
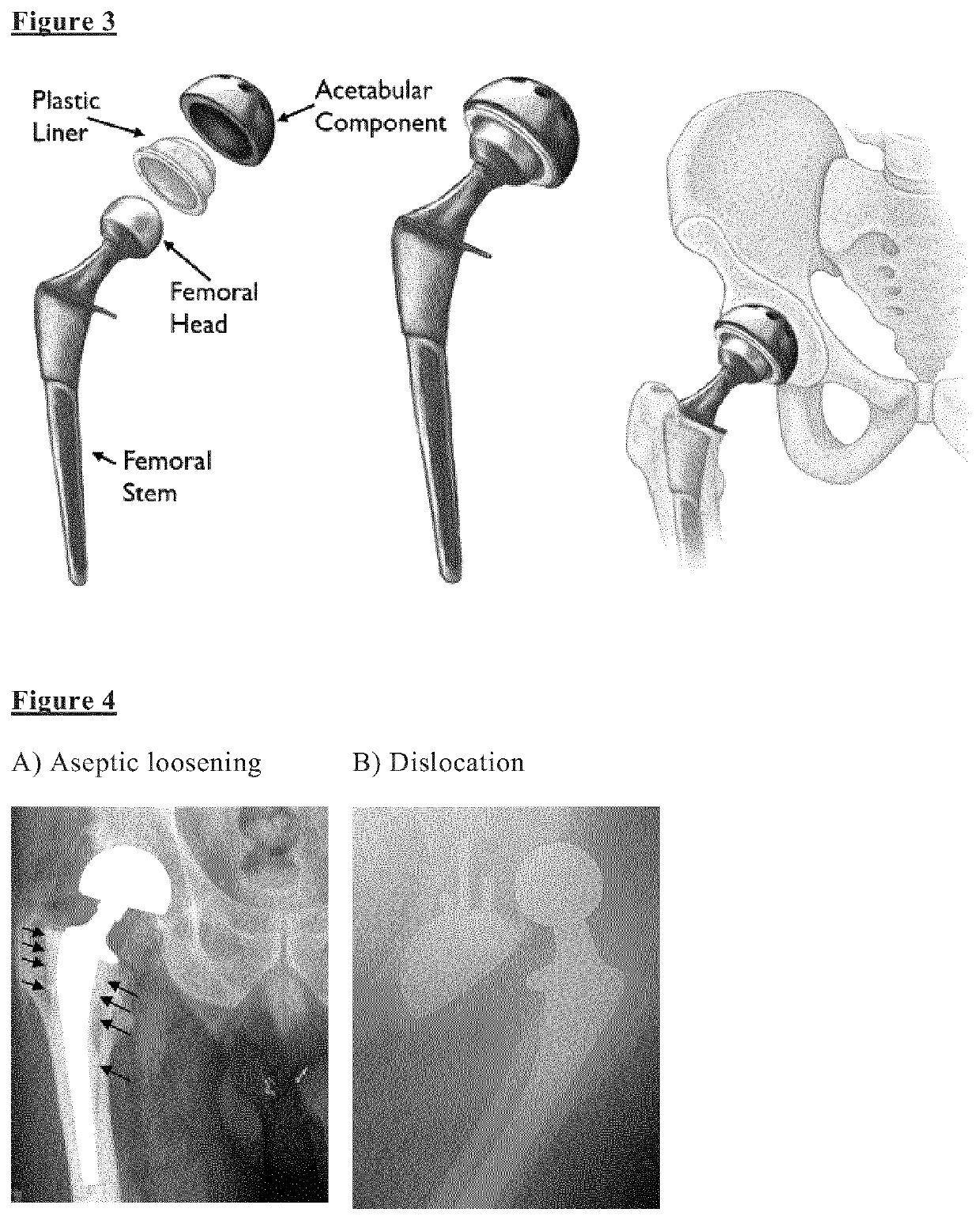
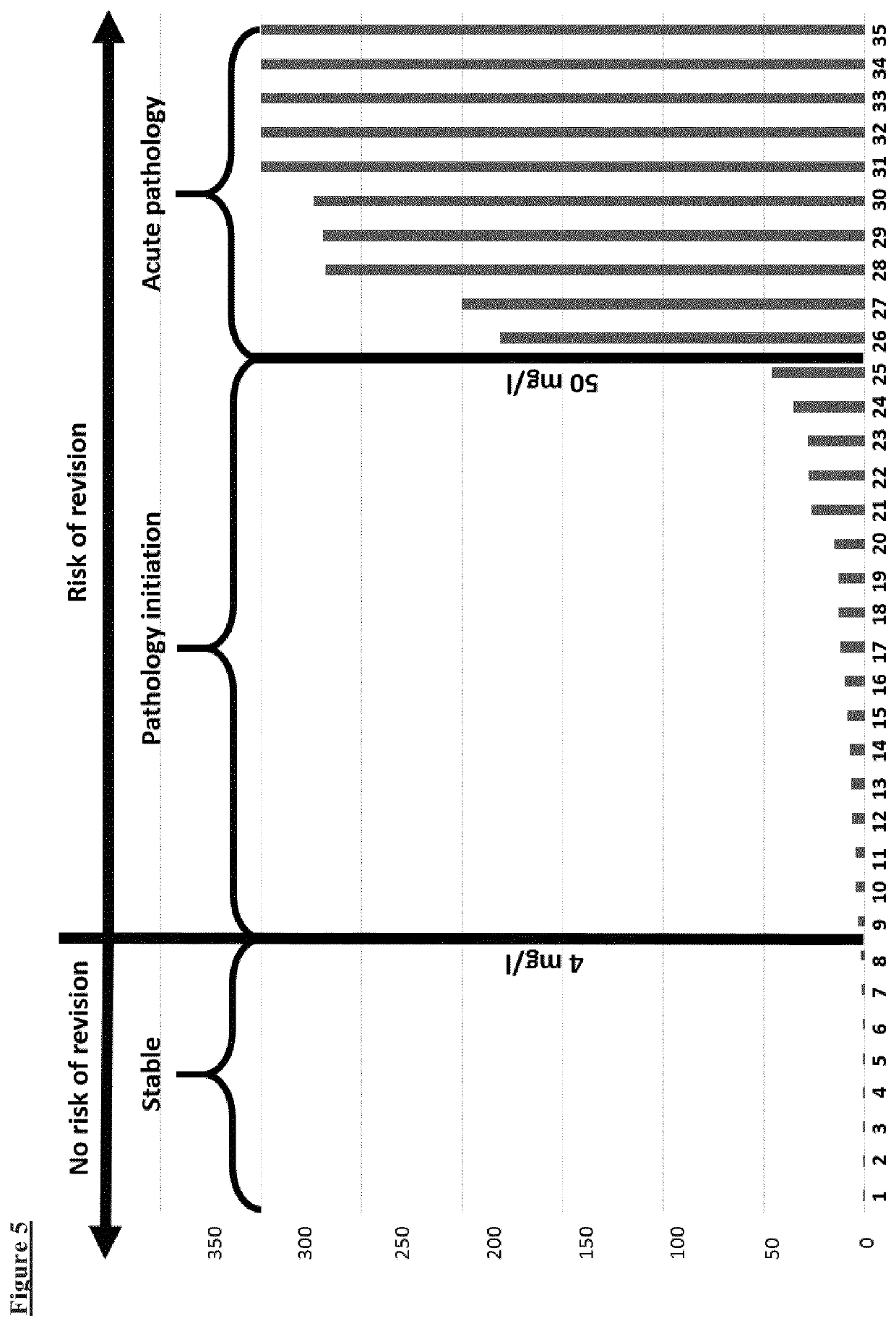
Biomarkers for diagnosing implant related risk of implant revision due to aseptic loosening
a biomarker and implant technology, applied in the field of implant related risk of revision, can solve the problems of difficult revision replacement, progressive bone destruction, and difficult revisions in the lifetime of younger patients
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
KIT for Measuring Levels of Calprotectin in a Sample
[0200]The CALPROLAB™ Calprotectin ELISA (ALP) is a quantitative assay for measuring Calprotectin in samples. The assay is commercially available.
[0201]Principle of the Test:
[0202]In the ELISA, samples and standards are incubated in separate microtiter wells coated with monoclonal antibodies which bind the Calprotectin. After incubation and washing of the wells, bound Calprotectin is allowed to react with enzyme-labelled, immunoaffinity-purified Calprotectin-specific antibodies. After this reaction, the amount of enzyme bound in the microtiter wells is proportional to the amount of Catprotectin in the sample or standard, which is determined by incubation with a substrate for the enzyme giving a coloured product. The colour intensity is determined by absorbance using an ELISA plate reader, and is proportional with the concentration of Calprotectin in the standards and samples. The assay is calibrated using Catprotectin purified from ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentration | aaaaa | aaaaa |
| concentration | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com