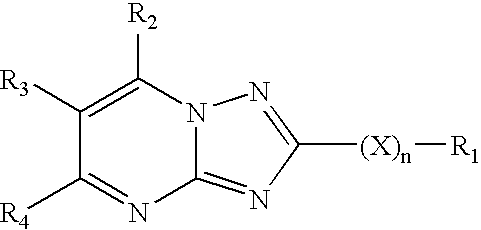
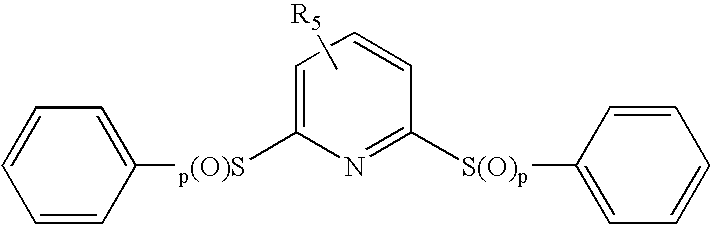
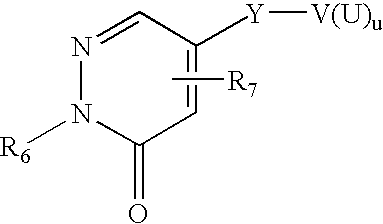
Affinity small molecules for the EPO receptor
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0139] The initial competitive ERP screening assay was designed as a solid plate binding assay. A detailed protocol of the assay is described below in the Protocolfor ERP Screen section.
[0140] Briefly, in the competitive ERP screening assay, the extracellular portion of EPO-R was purchased from R&D (Minneapolis, Minn.); biotinylated and regular peptide were synthesized by American Peptide Company; receptor and biotinylated peptide (bio-ERP) were incubated at room temperature; the solution was contacated with neutravidin (streptavidin) coated plate to bind any complex between receptor and peptide; the complex that was bound to the plate is detected by antibody specific for EPO-R and detection is performed with HRP-conjugated secondary antibody; the presence of the complex between receptor and peptide is detected by an light absorbance at 490 nm; and lack of or low signal indicates that the biotinylated peptide has been competed out, indicating an affinity of the test compound for the...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Volume | aaaaa | aaaaa |
| Volume | aaaaa | aaaaa |
| Volume | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com