Composite dosage forms
a technology of compound and dosage forms, which is applied in the direction of shaping presses, microcapsules, drug compositions, etc., can solve the problems of reducing the production efficiency of compound coating systems
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
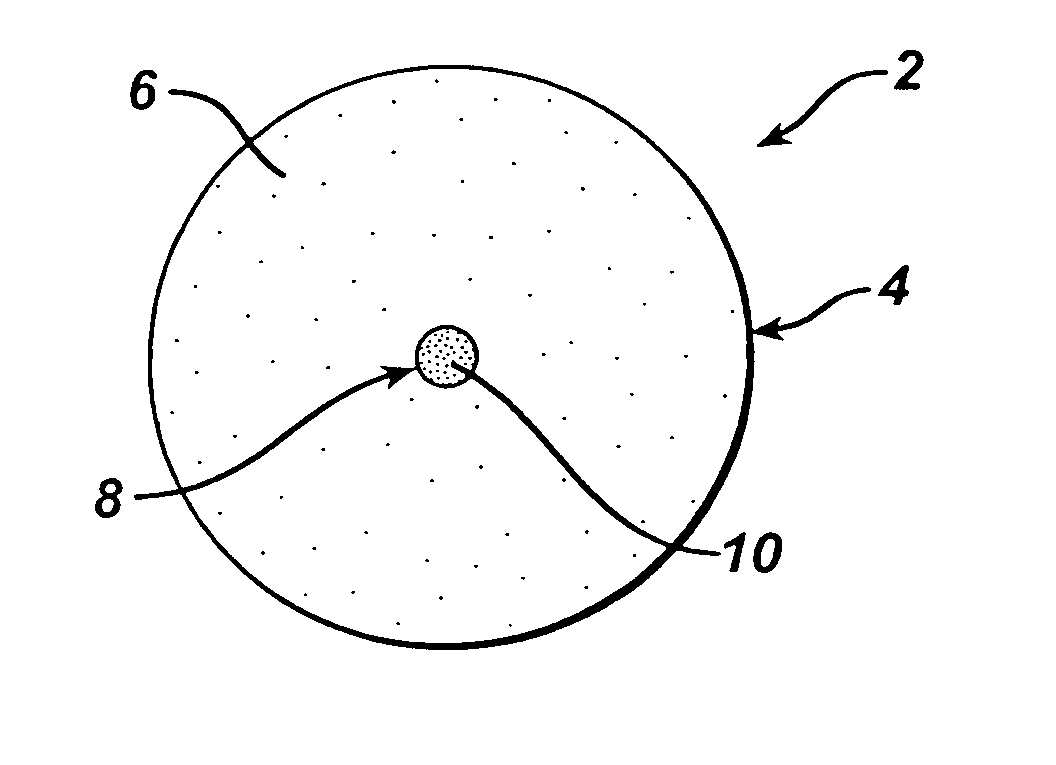
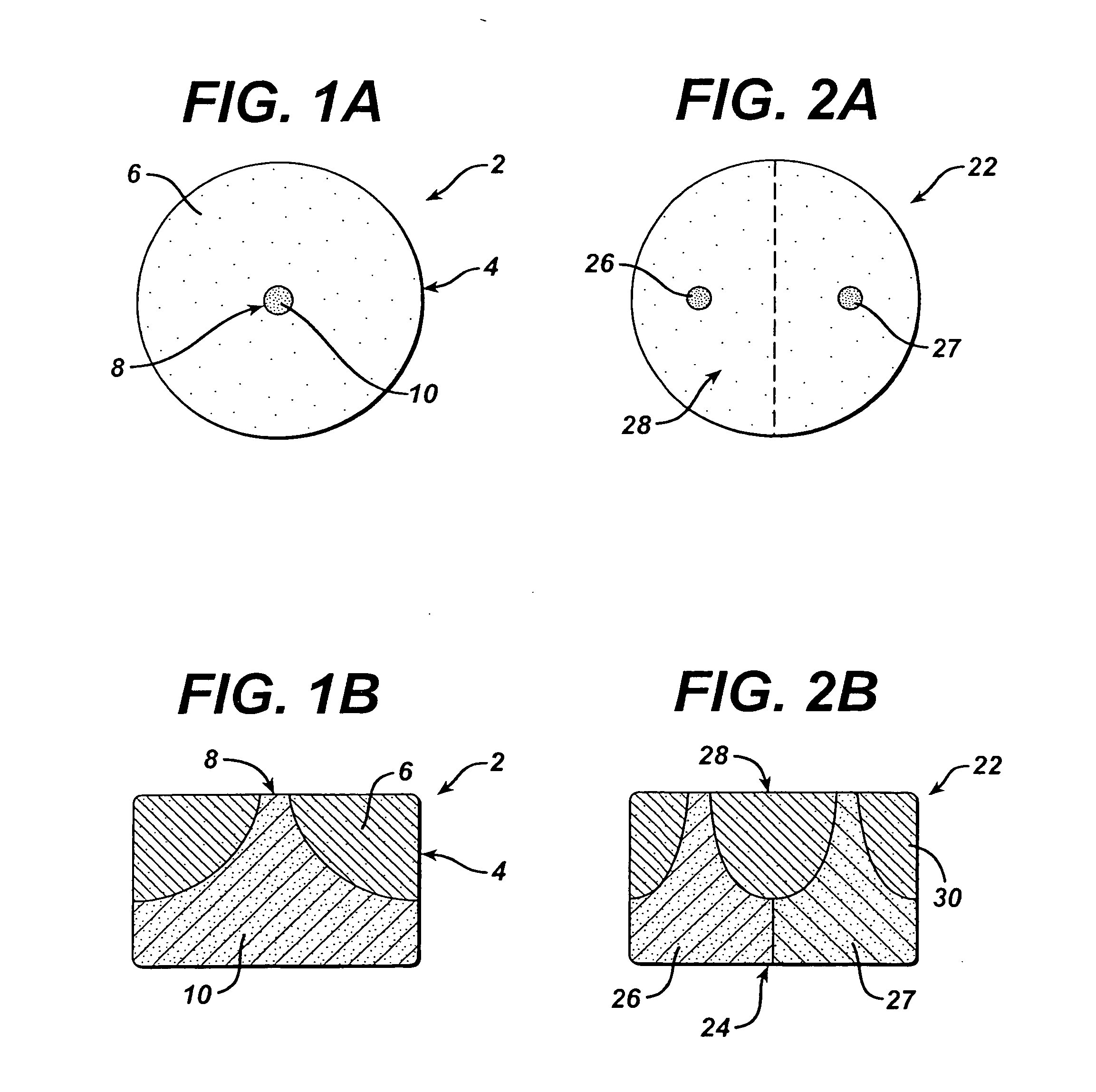
[0249] Dosage forms of the invention are made in a continuous process using an apparatus comprising a thermal cycle molding module and a compression module linked in series via a transfer device as described at pages 14-16 of copending U.S. application Ser. No. 09 / 966,939, the disclosure of which is incorporated herein by reference. The dosage forms have the structure shown in FIGS. 1A and 1B and comprise a first portion comprising a first molded material and a second portion comprising a second material that is compressed.
[0250] The first portions are made of a flowable material comprising the following ingredients:
WeightMg / TabletTrade NameManufacturer%TabletPolyethyleneCarbowax ®Union Carbide60.3190Glycol 3350Corporation,Danbury, CTCroscarmelloseAc-Di-Sol ®FMC Corporation,30.195SodiumNewark, DEPseudoephedrineBASF9.530HydrochloridePharmaChemikalienCrystalGmbH & Co.,Ludwigshafen / Rhein.
[0251] The second portions are made of a dry blend comprising the following ingredients: acetami...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| depth | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com