Delivery of oral drugs
a technology for oral administration and drugs, applied in the direction of pharmaceutical containers, packaging foodstuffs, packaged goods types, etc., can solve the problems of adversely affecting the release, stability and bioavailability of active ingredients, adverse effects on the release, and the use of excipients, etc., to achieve the effect of reducing was
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
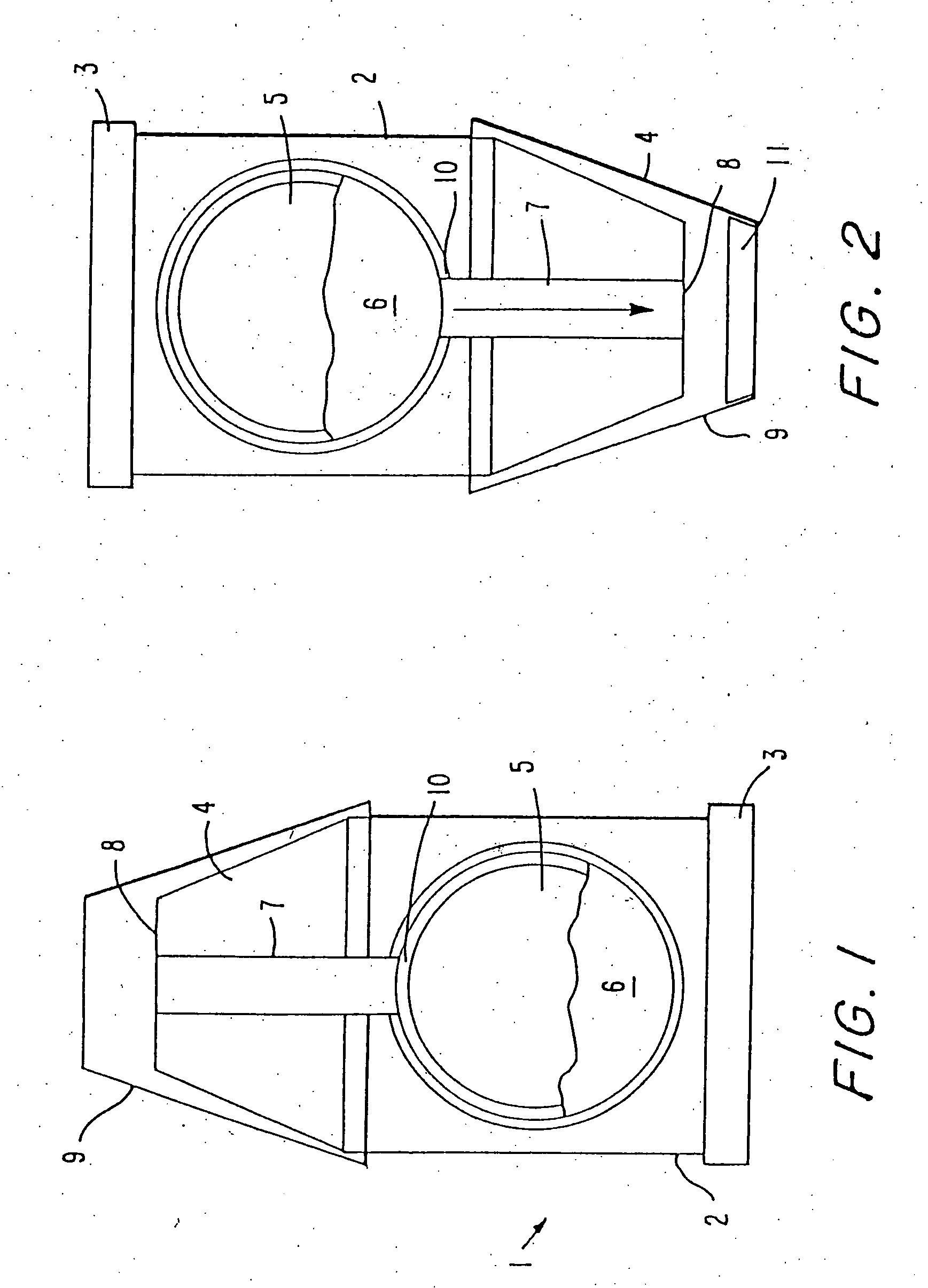
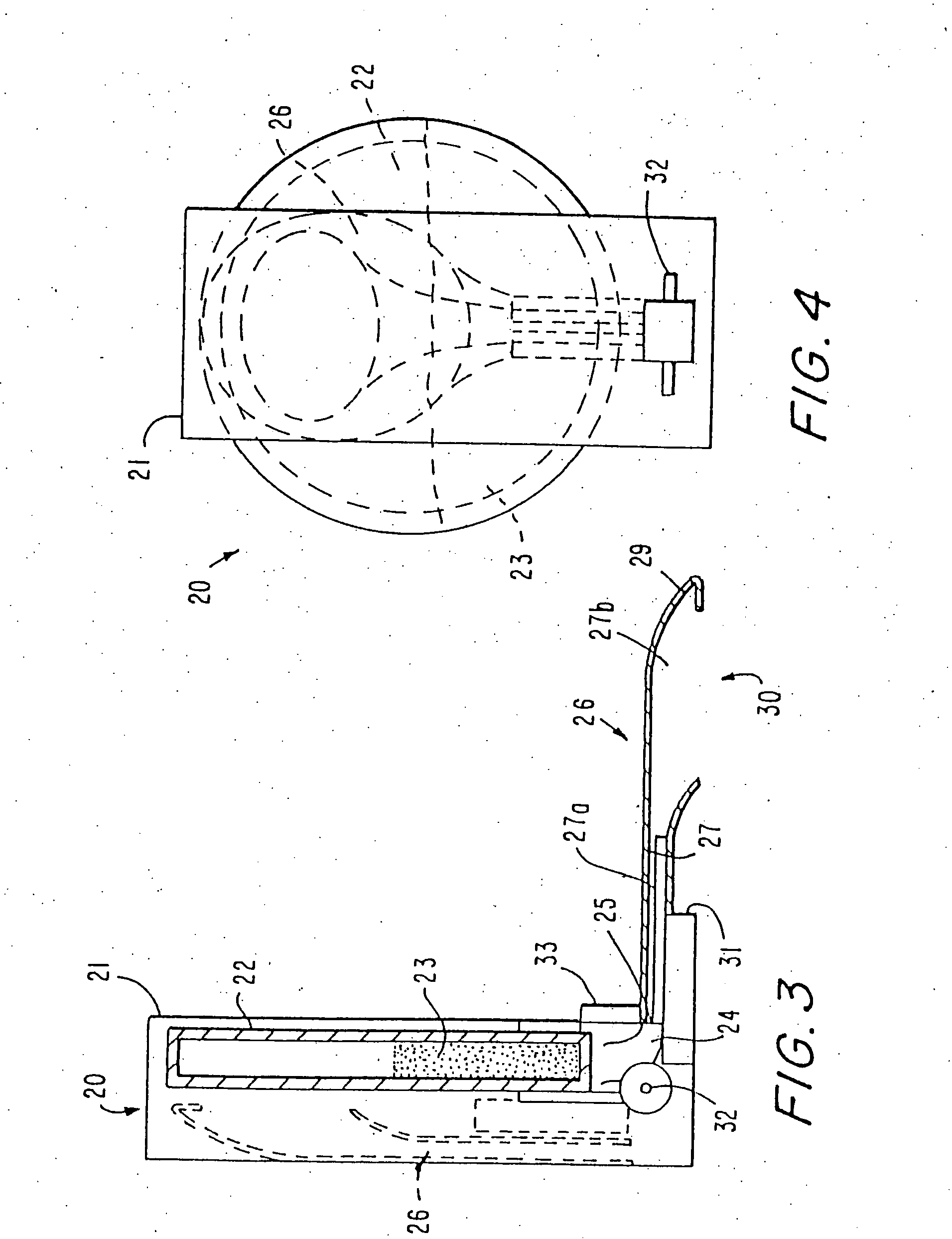
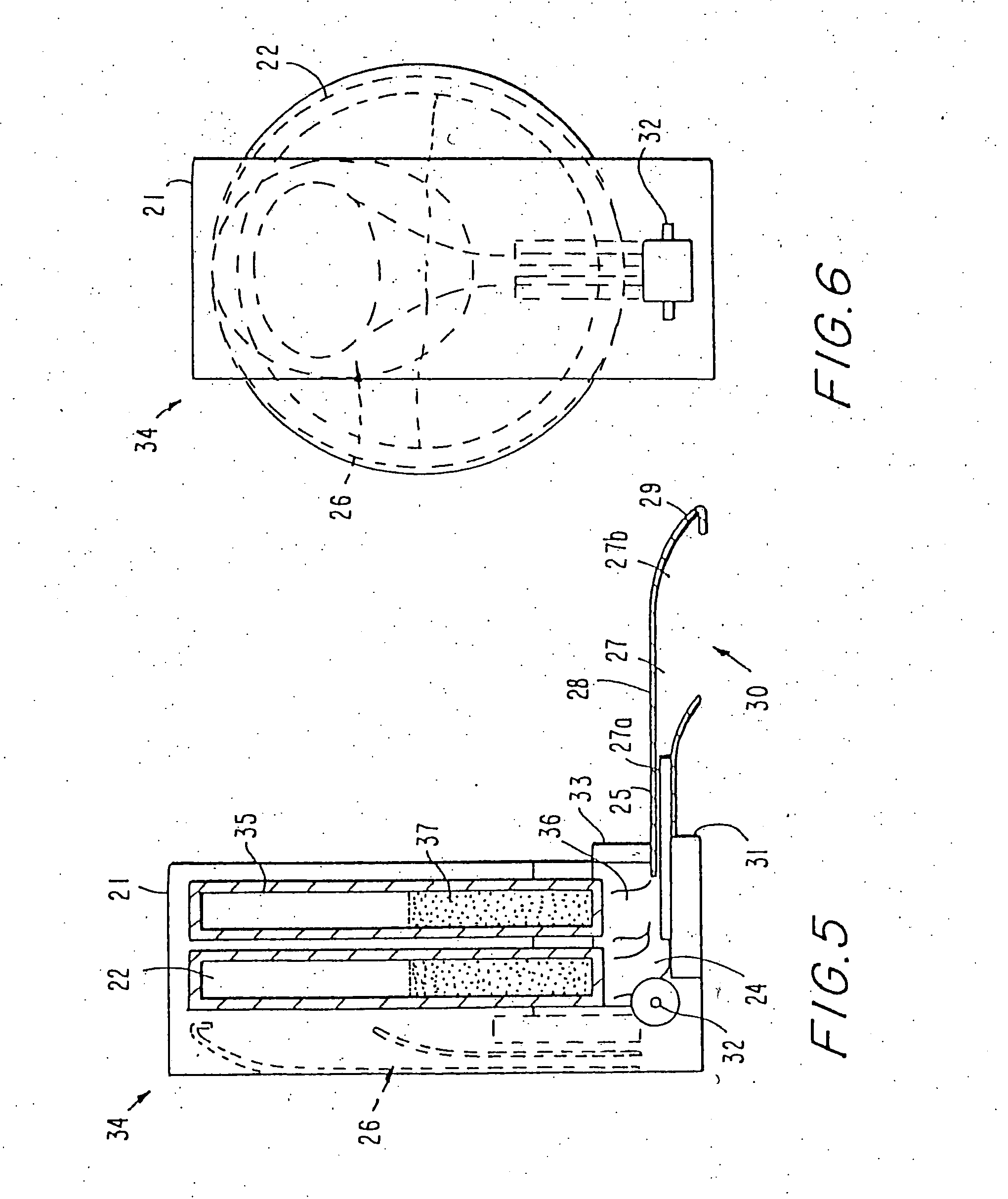
[0104] In general, it has been recognized in the art that dry powder inhalation or insufflation formulations must consist of particles of a size of about 2 microns in diameter in order for the particles, when inhaled, to reach the peripheral or “deep” lung, including alveoli. Particles larger than 10 microns in diameter are not able to reach the deep lung when inhaled because they are collected on the back of the throat and upper airways in humans. Therefore, known powder delivery systems have been formulated with particle sizes of less than 10 microns in order for the particles to reach the intended site of action, the pulmonary system. Known powder delivery devices have not contemplated delivery of particles from a multi-dose delivery device to achieve gastrointestinal deposition, and therefore have avoided the use of drug particles having a large size, e.g. greater than 10 microns. By virtue of the present invention, it has been a surprising discovery that drug particles greater ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| mean diameter | aaaaa | aaaaa |
| mean particle size | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com