DNA encoding tumor necrosis factor inhibitory protein and its use
a tumor necrosis factor and inhibitory protein technology, applied in the field of substantial purification of tumor necrosis factor inhibitory protein, can solve the problems of inability to always be advisable in humans, the effect of repetitive administration of murine monoclonal antibodies, and the effect of reducing the cytotoxic activity of tn
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
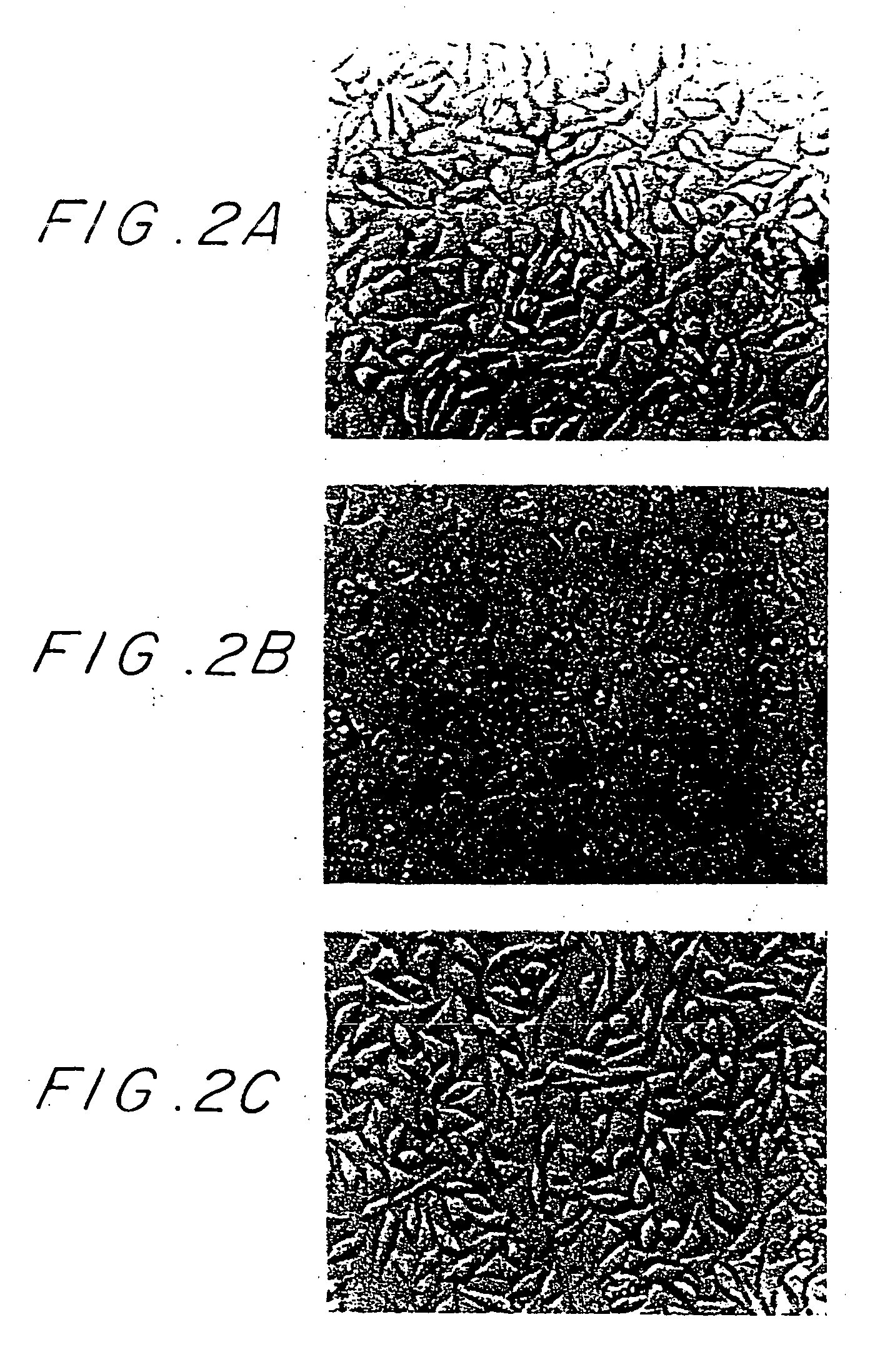
[0018] The present invention provides substantially purified TNF Inhibitory Protein and salts, functional derivatives and active fractions thereof, having the ability to inhibit the binding of TNF to its receptors and the cytotoxic effect of TNF.
[0019] It was found according to the present invention that the TNF Inhibitory Protein is able to inhibit the biological activities of both TNP-α and TNF-β and thus the inhibition of these two cytokines, herein referred to as TNF, by the TNF Inhibitory Protein, is encompassed by the present invention.
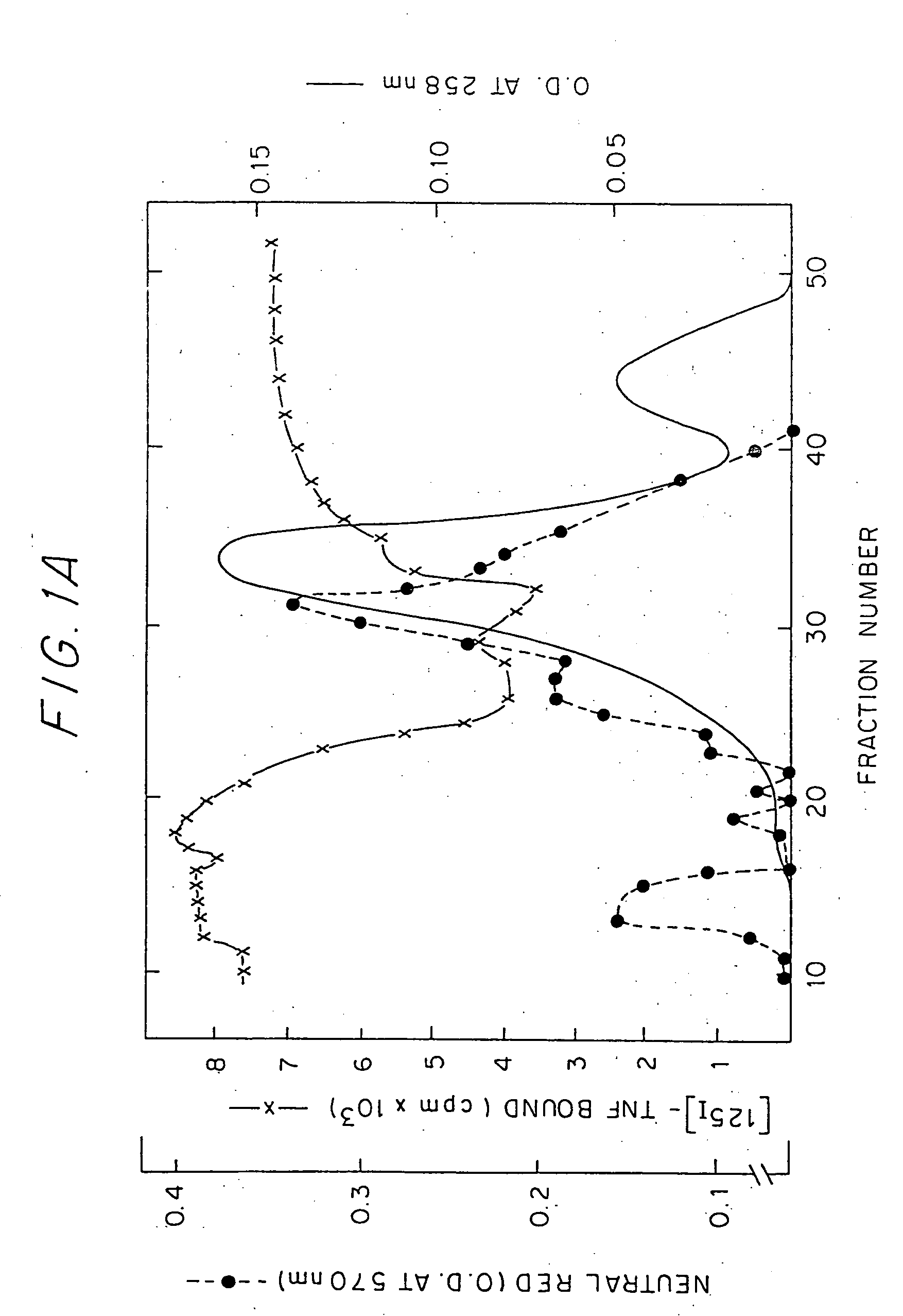
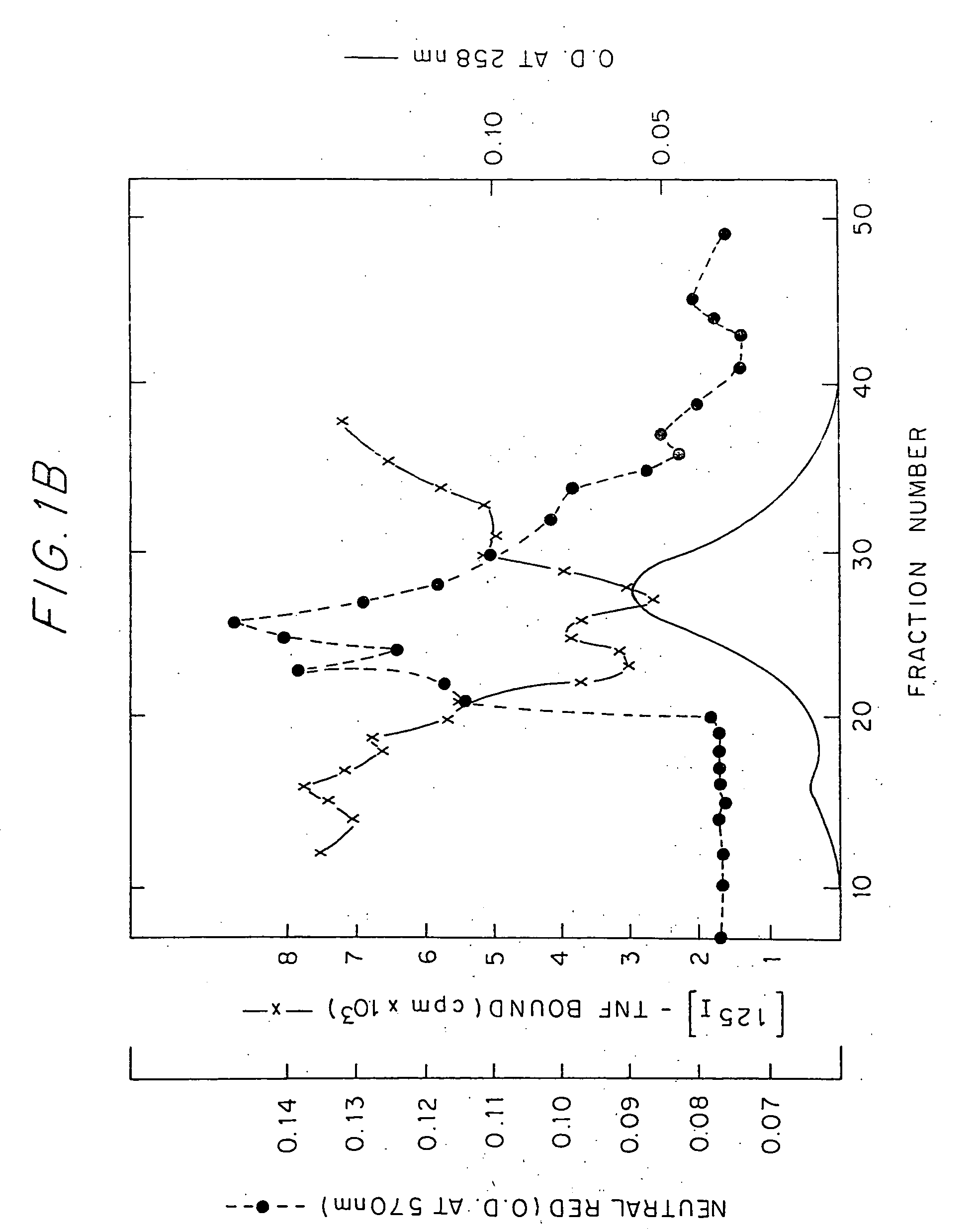
[0020] The TNF Inhibitory Protein of the invention may be found in human urine. When crude preparations thereof derived from human urine concentrate were chromatographed on Ultrogel ACA 44 gal filtration column, it showed an apparent molecular weight of 40-80 kDa. The substantially purified protein, which le substantially free of proteinaceous impurities, has a molecular weight of about 26-28 kDa when analyzed by SDS PAGE under reducing condit...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Fraction | aaaaa | aaaaa |
| Fraction | aaaaa | aaaaa |
| Fraction | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com