A kind of tripterine derivative and its preparation method and application
A technology of tripteryglide and its derivatives, which is applied in the direction of medical formulas, drug combinations, steroids, etc., can solve the problems of tripterygine's insufficient druggability, no significant improvement in anti-tumor activity, etc., and achieve the reduction of cytotoxic activity , Increase the effect of water solubility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
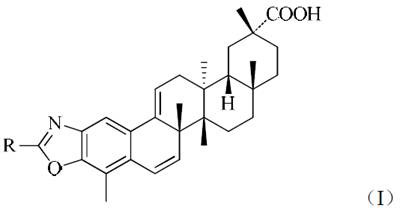
[0032] Embodiment 1: the synthesis of tripterine derivative Ia
[0033]
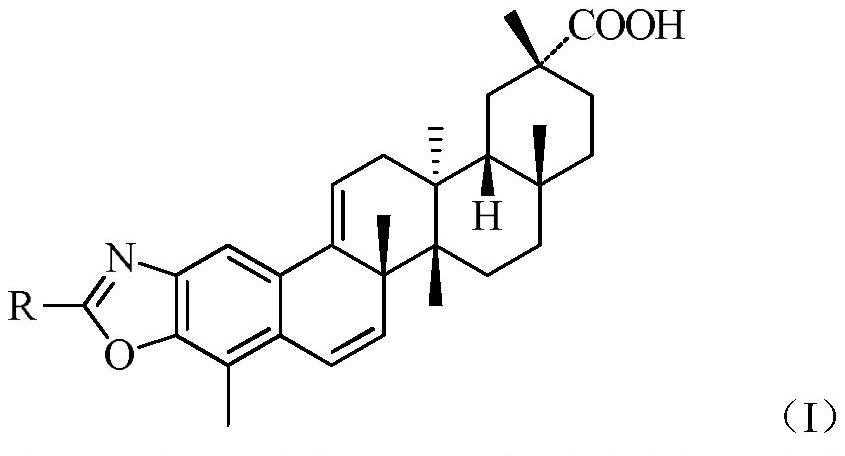
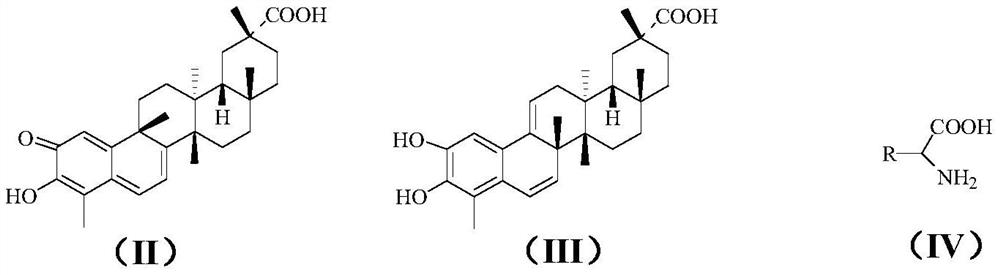
[0034] Methanol (4mL) and tripterine (45.0mg, 0.1mmol) were added to a 25mL two-neck flask, and hydrochloric acid (1mL) at pH=1 was added with stirring at room temperature, and the pH of the reaction solution was=1-2. React at room temperature for 12 hours, add glycine (15.0mg, 0.2mmol), add sodium carbonate to adjust pH = about 8, continue to react for 8 hours, TLC detection, after the reaction, add water (5mL), stir to dissolve the sodium carbonate solid, Add hydrochloric acid to make the pH of the reaction solution around 7, extract three times with ethyl acetate (10 mL), combine the organic phases, wash twice with saturated brine, dry over anhydrous magnesium sulfate, then suction filter, and concentrate the filtrate to dryness to obtain crude product. The crude product was separated and purified by flash column chromatography (petroleum ether: ethyl acetate = 6:1), and the product was vacuum-dri...
Embodiment 2
[0037] Embodiment 2: the synthesis of tripterine derivative Ib
[0038]
[0039] Methanol (4mL) and tripterine (45.0mg, 0.1mmol) were added to a 25mL two-necked bottle, and hydrochloric acid (1mL) with pH=1 was added under stirring at room temperature, and the pH of the reaction solution was about 1-2. React at room temperature for 12 hours, add alanine (17.8mg, 0.2mmol), add sodium carbonate to adjust pH=8, continue to react for 8 hours, TLC detection, after the reaction, add water (5mL), stir to dissolve sodium carbonate solid, add hydrochloric acid to make the pH of the reaction solution around 7, extract three times with ethyl acetate (10 mL), combine the organic phases, wash with saturated brine, and then dry with anhydrous magnesium sulfate, then suction filter, and the filtrate is concentrated to dryness to obtain crude product. The crude product was separated and purified by flash column chromatography (petroleum ether: ethyl acetate = 6:1), and the product was vac...
Embodiment 3
[0042] Embodiment 3: the synthesis of tripterine derivative Ic
[0043]
[0044] Methanol (4mL) and tripterine (45.0mg, 0.1mmol) were added to a 25mL two-necked bottle, and hydrochloric acid (1mL) with pH=1 was added under stirring at room temperature, and the pH of the reaction solution was about 1-2. React at room temperature for 12 hours, add valine (23.4mg, 0.2mmol), add sodium carbonate to adjust pH=7, continue to react for 8 hours, TLC detection, after the reaction, add water (5mL), stir to dissolve sodium carbonate solid, add hydrochloric acid to make the pH of the reaction solution = about 7, extract three times with ethyl acetate (10 mL), combine the organic phases, wash twice with saturated brine, dry over anhydrous magnesium sulfate, and then filter with suction, and concentrate the filtrate to dryness to obtain crude product. The crude product was separated and purified by flash column chromatography (petroleum ether: ethyl acetate = 6:1), and the product was v...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com