Refrigerator-temperature stable influenza vaccine compositions
a technology of influenza vaccine and composition, which is applied in the direction of drug composition, antibody medical ingredients, immunocompromised persons, etc., can solve the problems of delayed and shortage of production and distribution, special risk of death for immunocompromised persons, and elderly people without adequate health car
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Development of Liquid FluMist
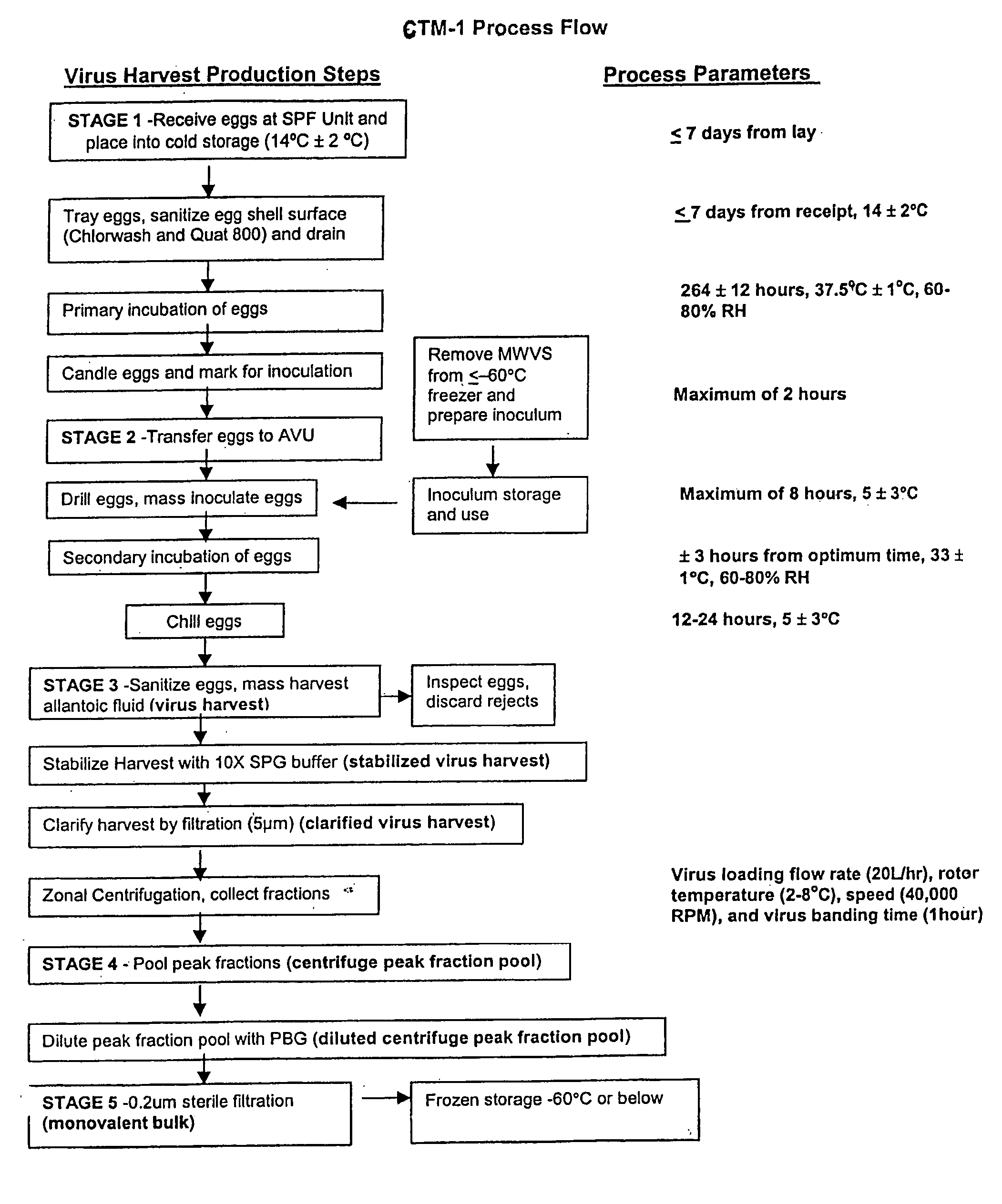
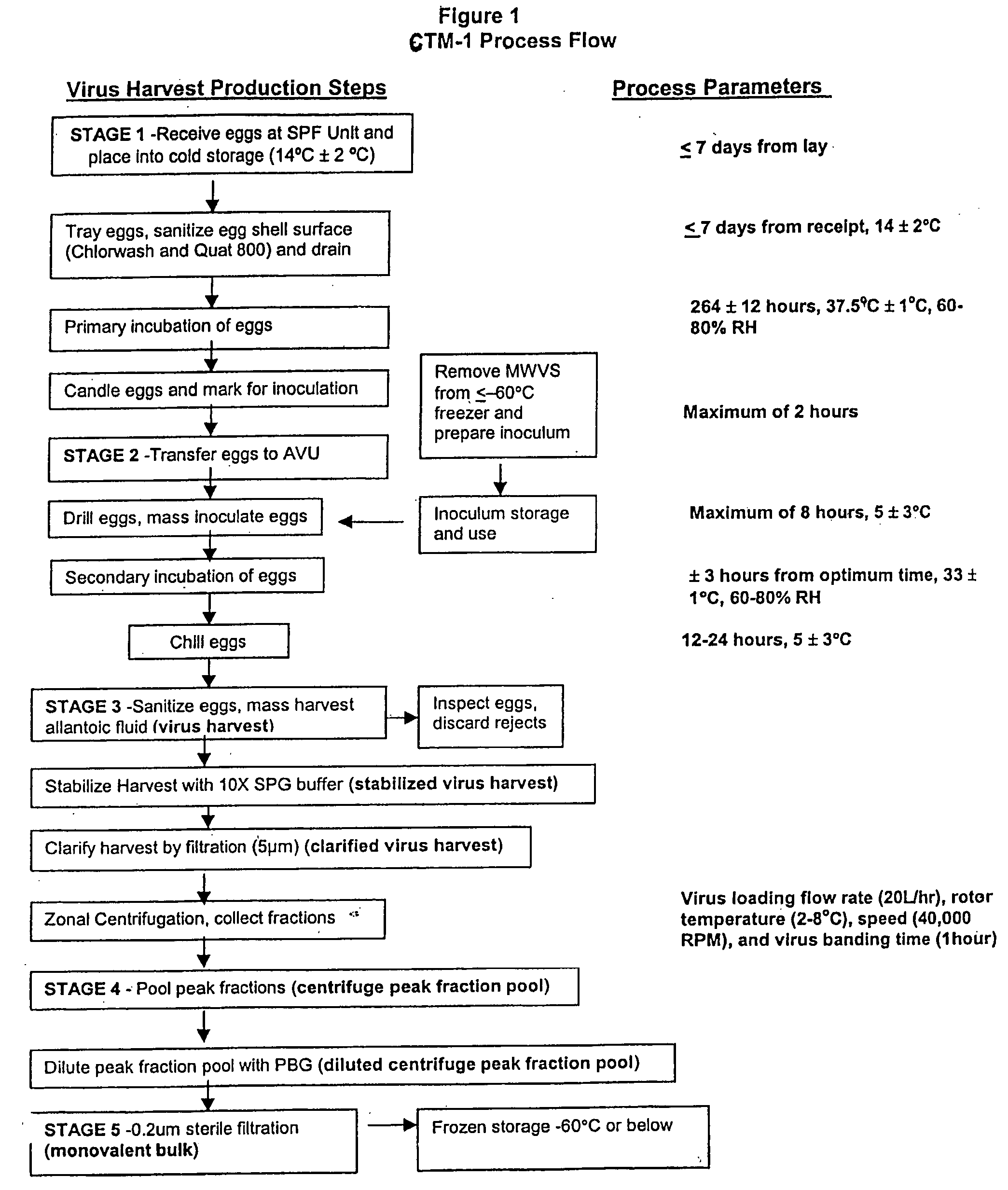
[0166] A total of 19 monovalent bulk lots of virus were initiated under protocol, including four development lots (CB0006H, CB0008H, CG0017H, CB0018H and CB0019H). Lots CB0018H and CB0019H were conducted to supply material for various studies and are not discussed further in this application. Two lots (CB00180H and CB0012H) were combined after the ultra-centrifugation step to produce A / Sydney monovalent bulk CB0020H. Each lot was initiated with about 2000 eggs.
[0167] Egg handling and incubation conditions were set up similar to the process used for production of frozen FluMist, however, manual inoculation and harvesting were used due to the smaller scale, etc. The ultracentrifuge process was scaled down to smaller equipment made by the same manufacturer (Discovery 90, made by Hitachi and marketed by Sorvall / heraus), with a Model P32CT rotor having approximately 470 mL of total capacity. Clarified, stabilized virus harvest was loaded onto a 20% to 60% ...
example 2
Stability Testing
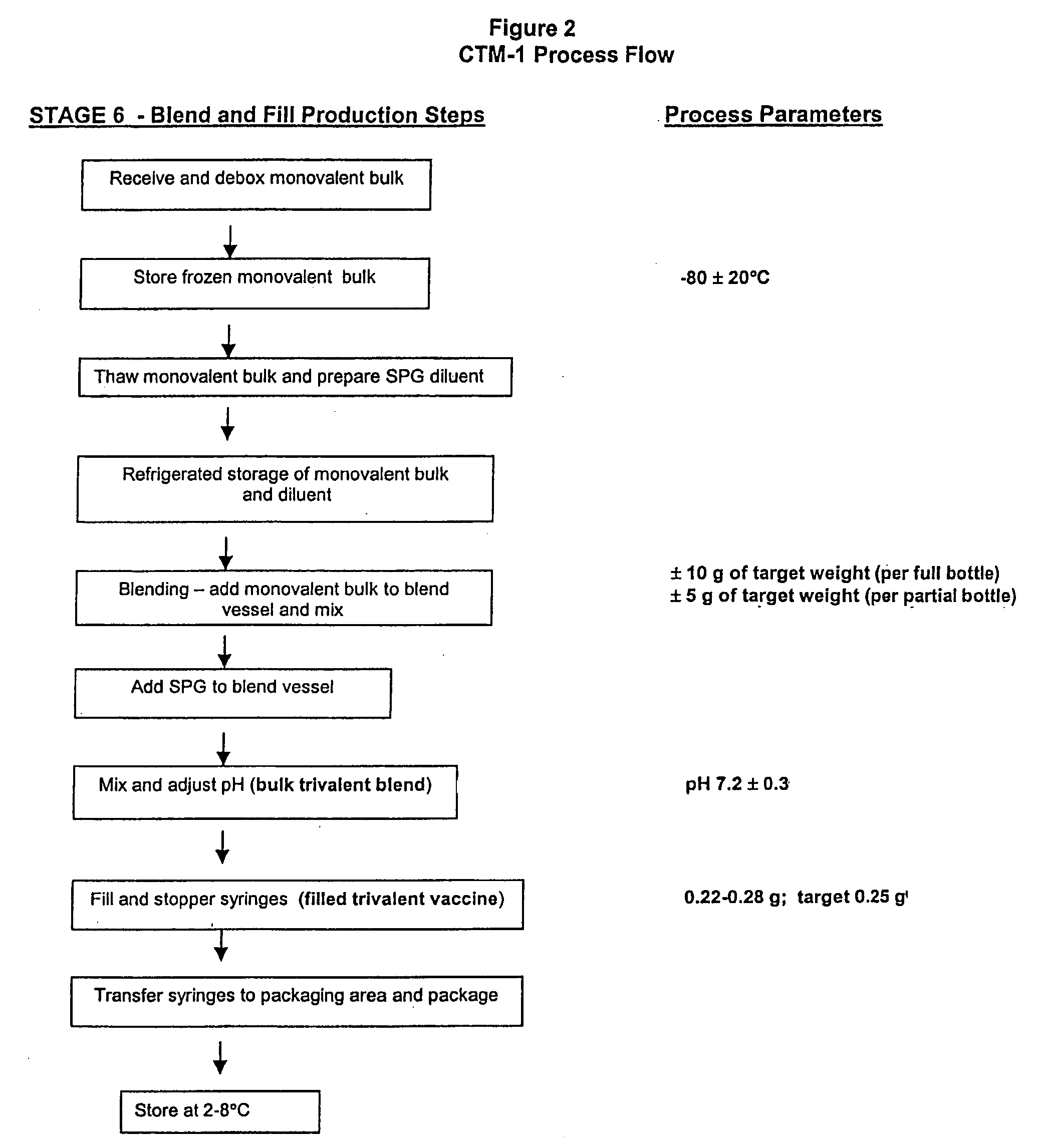
[0233] A trivalent vaccine formulation was prepared comprising three different reassortant influenza viruses (7.0+ / −0.5 log10 FFU / dose [approximately 7.0+ / −0.5 log10TCID50 / dose]) and comprising 200 mM sucrose; 1% (w / v) porcine gelatin hydrolysate; 1.21% (w / v) arginine monohydrocloride [equivalent to 1% (w / v) arginine base]; and 5 mM monosodium glutamate in 100 mM potassium phosphate buffer (pH 7.2). The stability of the formulation was stored at −25.0 degrees C+ / −5.0 degrees for greater than or equal to 24 hours, but less than or equal to two weeks and then stored at 2-8 degrees C. for various time periods. This formulation (e.g., lot 0141500003) was determined to be stable for at least 12 weeks at 4-8 degree C. In particular, the potency for each strain of virus remained within 0.5 log10 of the beginning potency prior to 4-8 degree C. storage.
[0234] Other studies have shown that equivalent formulations (comprising three different reassortant influenza viruses (7....
PUM
| Property | Measurement | Unit |
|---|---|---|
| Temperature | aaaaa | aaaaa |
| Temperature | aaaaa | aaaaa |
| Fraction | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com