Cell lysis composition, methods of use, apparatus, and kit
a cell lysis and composition technology, applied in the field of cell lysis composition, methods for extracting and purifying proteins, apparatuses and kits, can solve the problems of additional costly and time-consuming purification steps for the removal of contaminants
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Preparation of Cell Lysis Reagent
[0145] In this Example, several representative cell lysis reagents are described. For cell pellets, a cell lysis reagent at 1× concentration aqueous formulation is preferably used. For cell media, a cell lysis reagent at 10× concentration is preferable.
(a) Cell Lysis Reagent at 1× Concentration:
[0146] This 1× aqueous formulation is useful for extracting proteins or peptides from cell pellets (frozen or non-frozen). The amount of the formulation added to the pellets is generally based on the optical density of the cells. For example, 200 ul of 1× formulation is used for the lysis of cells with an OD600 of 1.8 / 1 ml. The formulation contains the following components:
100 mM HEPES, pH 7.5,
1% Triton X-100 (Sigma, St. Louis, Mo., Cat#T-9284)
1% Mazu DF204 (defoaming agent, PPG Industries, Gurnee, Ill., Cat#213306-2)
0.4% Tomah (purified Tomah E-18-15, Bioaffinity systems, Roscoe, Ill., Cat#016483)
10 mM imidazole (Sigma, St. Louis, Mo.; Cat#I-239...
example 2
Release of Renilla Luciferase from E. coli
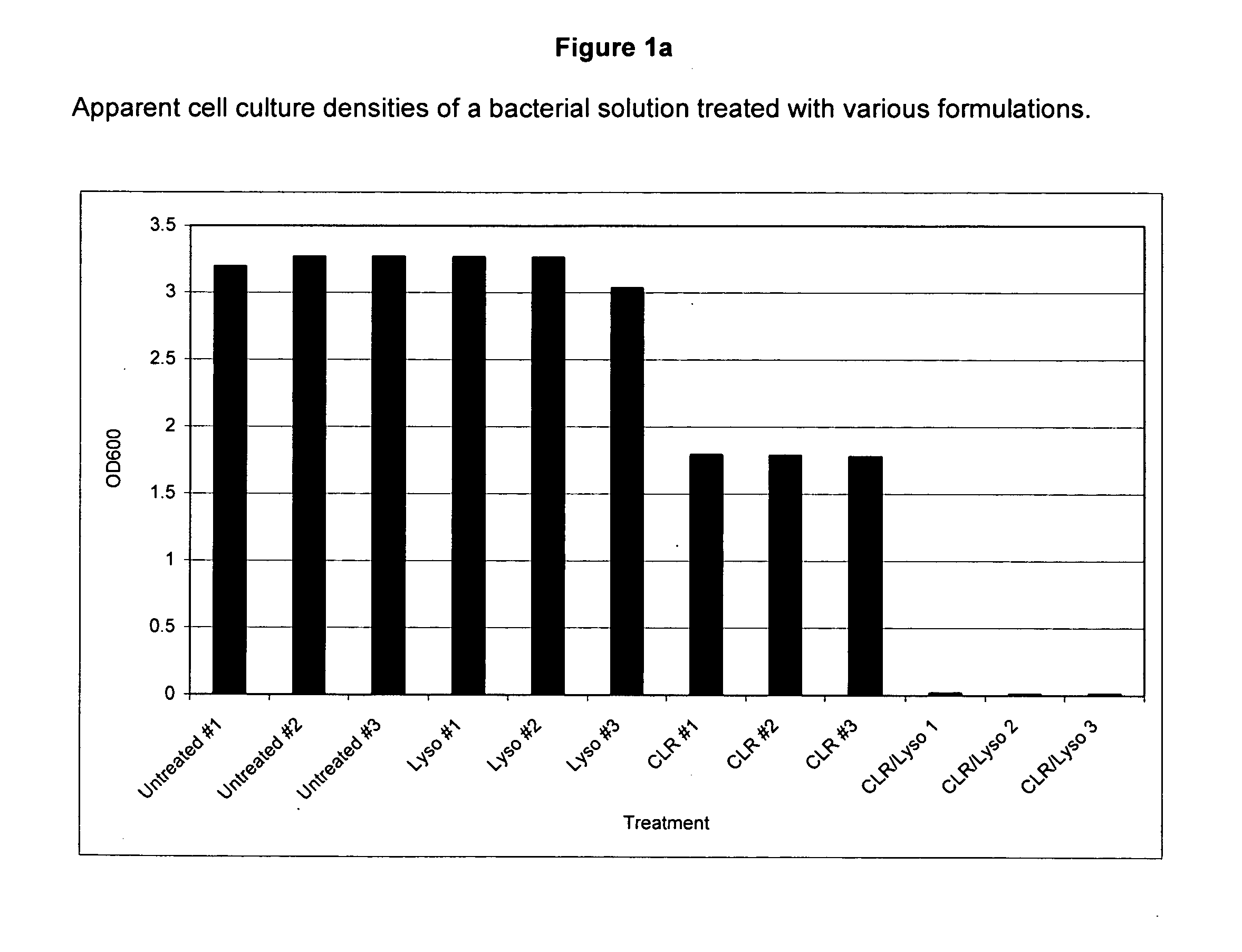
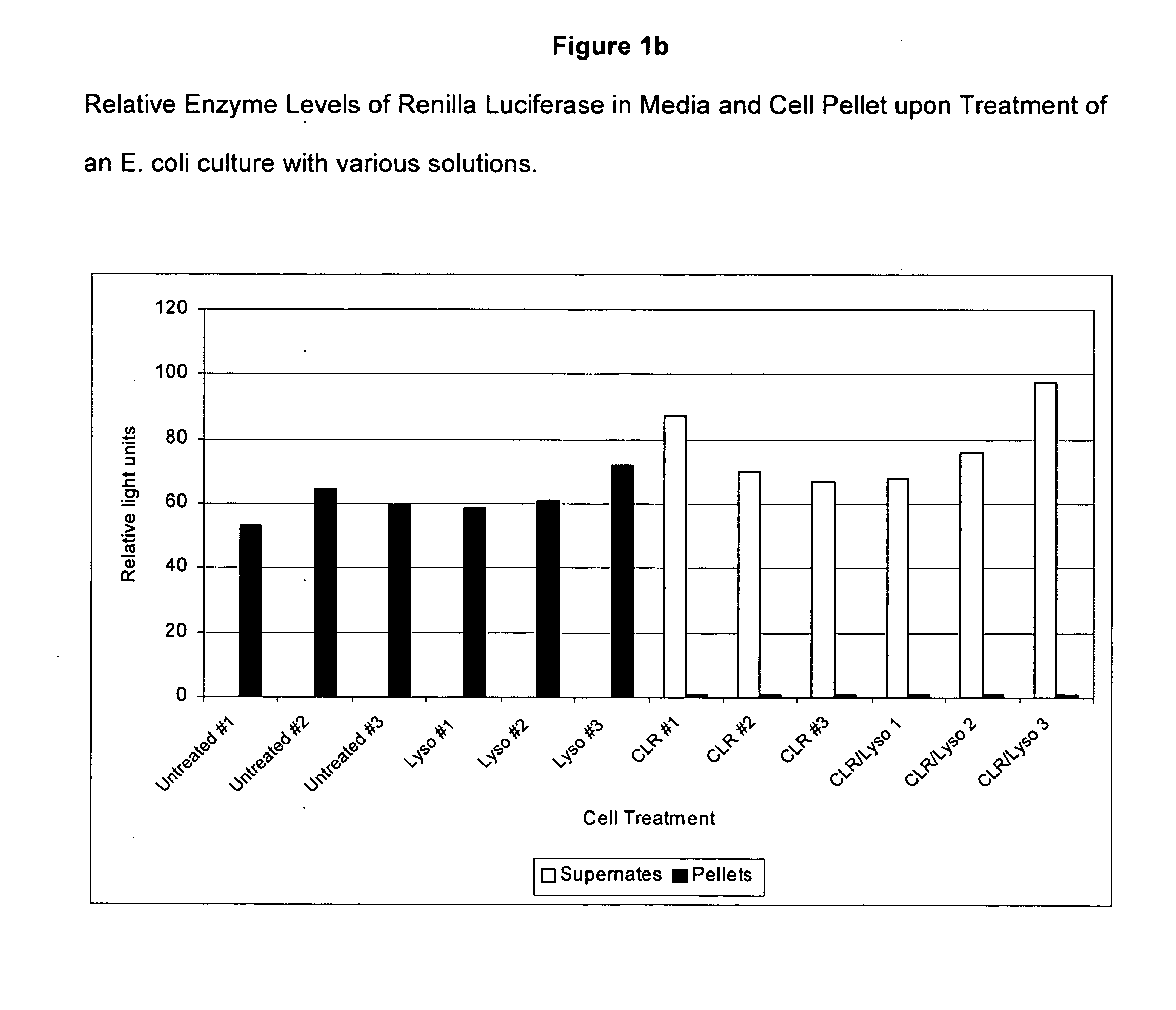
[0148] Cytoplasmic protein, as measured by the enzyme Renilla Luciferase, is released from E. coli cells when the cells are treated with a solution containing detergent and Polymyxin B. Surprisingly, this release of enzyme is not accompanied by general cell lysis as measured by observation of the optical density of the culture during the treatment.
[0149]E. coli bacteria expressing His-tagged Renilla Luciferase were grown in Luria Broth [L Broth]+10 ng / ml tetracycline [Tet] [50 ml of media in a 250 ml flask] at 37° C. overnight in a shaking incubator rotating at 200 RPM. The E. coli strain was prepared by transforming E. coli with a vector expressing histidine-tagged Renilla luciferase. The vector was constructed by conventional methods. See Maniatis et al., “Molecular Cloning: A Laboratory Manual,” 2nd Edition, Cold Spring Harbor Laboratory, Cold Spring Harbor, N.Y. (1982). Following overnight culture the bacterial cells were diluted 1:100...
example 3
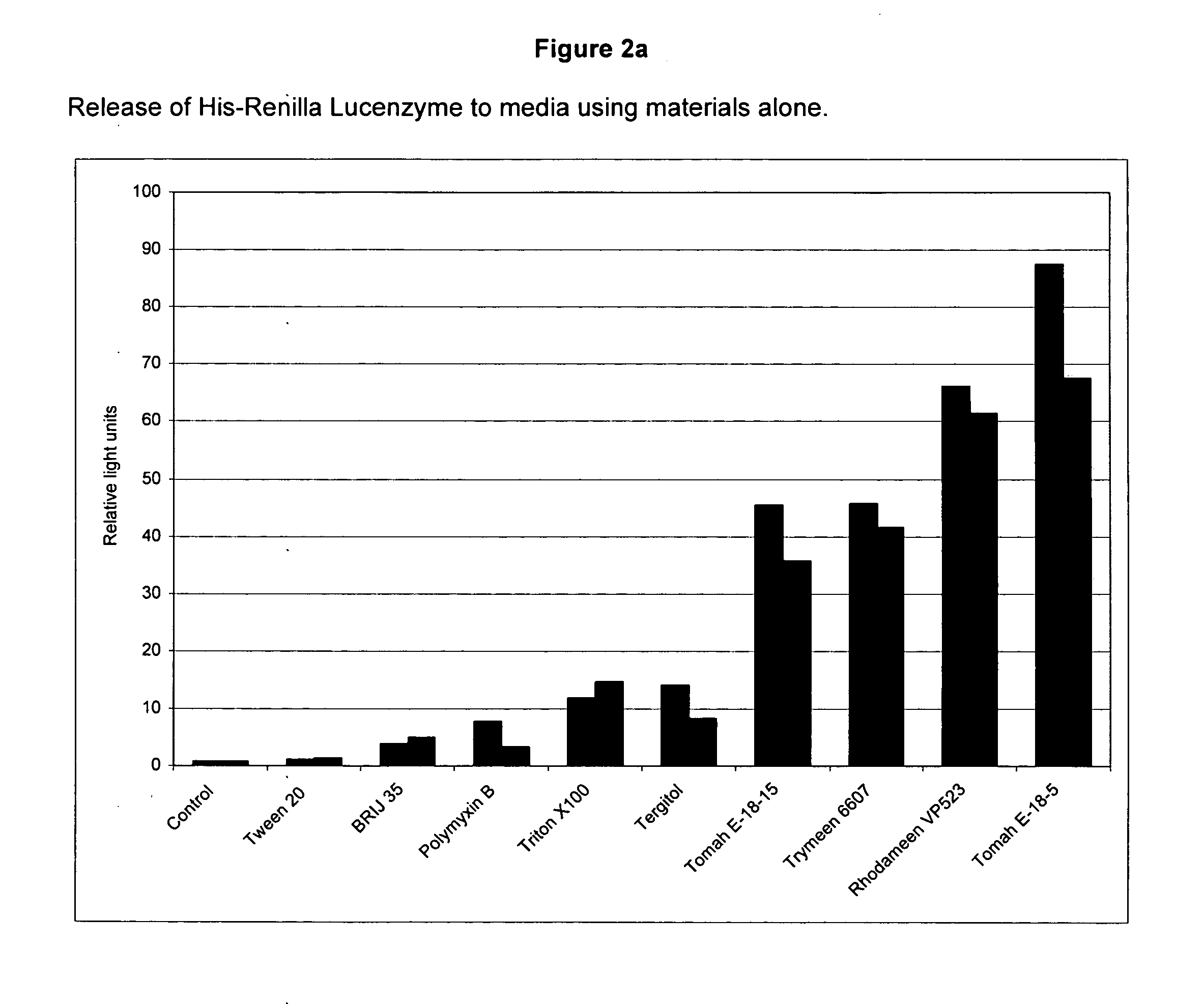
Screening of Detergents for the Ability to Release Enzymes into Media from E. coli Without Inactivation of the Enzyme
[0158] In this example, a number of detergents were tested for their ability to release cytoplasmic protein from E. coli cells alone and in combination with Polymyxin B.
[0159] The following stock detergent solutions were prepared:
Two grams of deoxycholic acid, sodium salt [Sigma D 6750, 102H0811] was dissolved in deionized water to produce a 4% (v / v) DOC solution.
Two grams of Lauryl Sulfate, sodium salt [Sigma L 4390, 73H0057] was dissolved in deionized water to produce a 4% (v / v) SDS solution.
Two grams of Tomah E-14-5 [Tomah Chemical Company, lot 71002-1] was dissolved in deionized water to produce a 4% (v / v) Tomah E-14-5 solution.
Two grams of Tomah E-14-2 [Tomah Chemical Company, lot 70224-1] was dissolved in deionized water to produce a 4% (v / v) Tomah E-14-2 solution.
Two grams of Tomah E-18-15 [Tomah Chemical Company, lot 60911-1] was dissolved in deion...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Fraction | aaaaa | aaaaa |
| Fraction | aaaaa | aaaaa |
| Fraction | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap