Synthesis of acenes and hydroxy-acenes
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
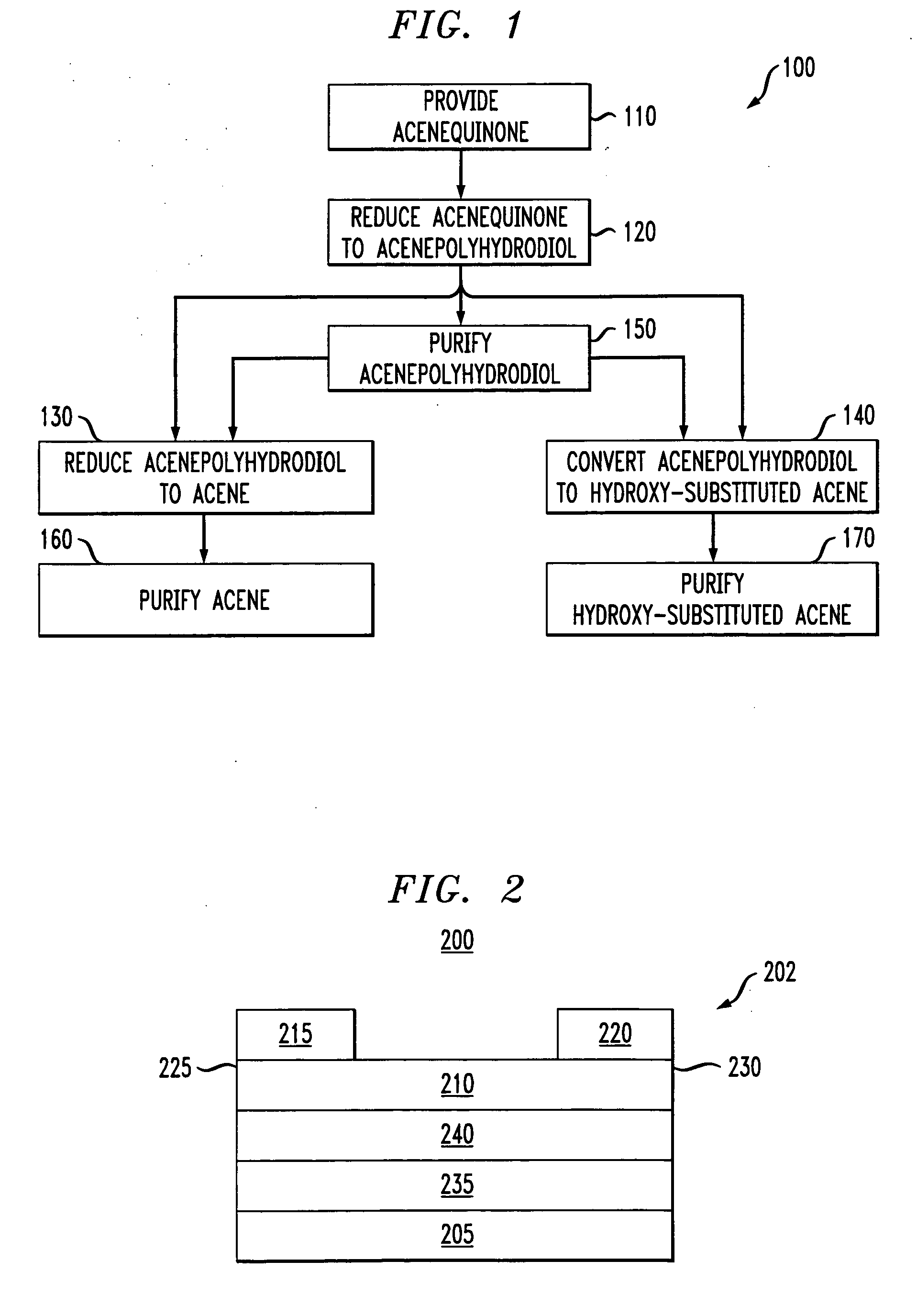
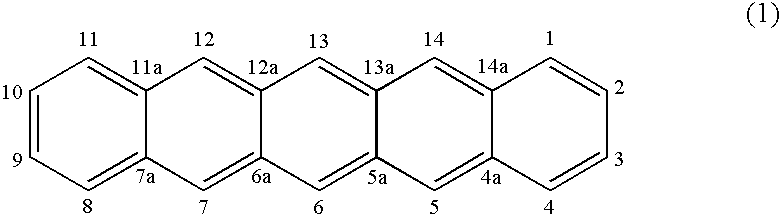
[0012] The present invention recognizes the benefits of synthesizing acenes via two consecutive reduction reactions. In the first reduction reaction, an acenequinone of the target acene is converted into an acenepolyhydrodiol. In the second reduction reaction, the acenepolyhydrodiol can be converted to the target acene. Alternatively, the acenepolyhydrodiol can be thermally converted into a hydroxy-substituted acene. These reactions advantageously reduce the need for toxic compounds for the synthesis. In addition, some of the acenepolyhydrodiols or hydroxy-substituted acenes are soluble in organic solvents, thereby facilitating the fabrication of devices via solution deposition processes.
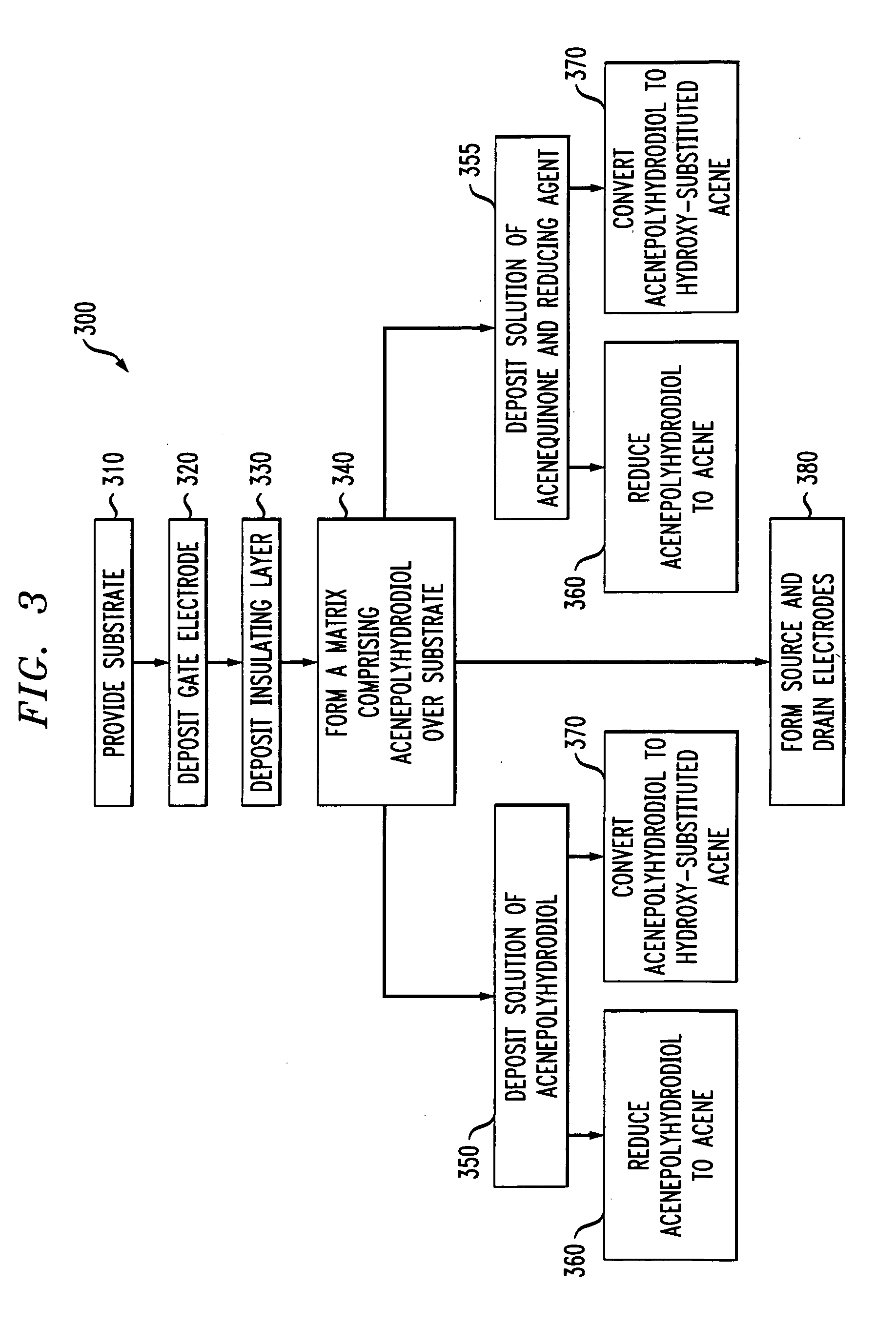
[0013] One embodiment is a method of synthesis. FIG. 1 presents a flow diagram showing selected steps in an exemplary method of synthesis. In step 110, an acenequinone of the target acene is provided. In step 120, the acenequinone is reduced to form an acenepolyhydrodiol by exposing the acenequinon...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Temperature | aaaaa | aaaaa |
| Semiconductor properties | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com