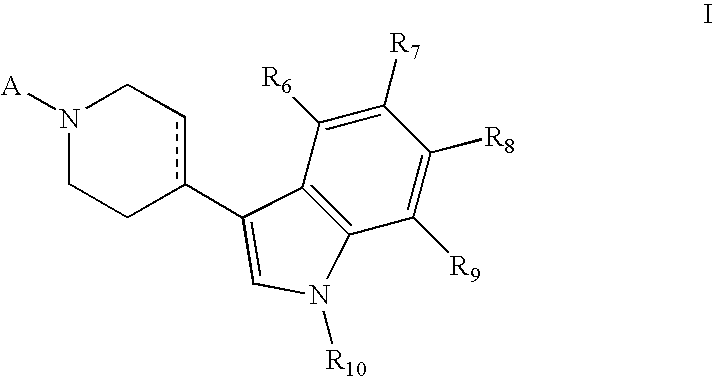
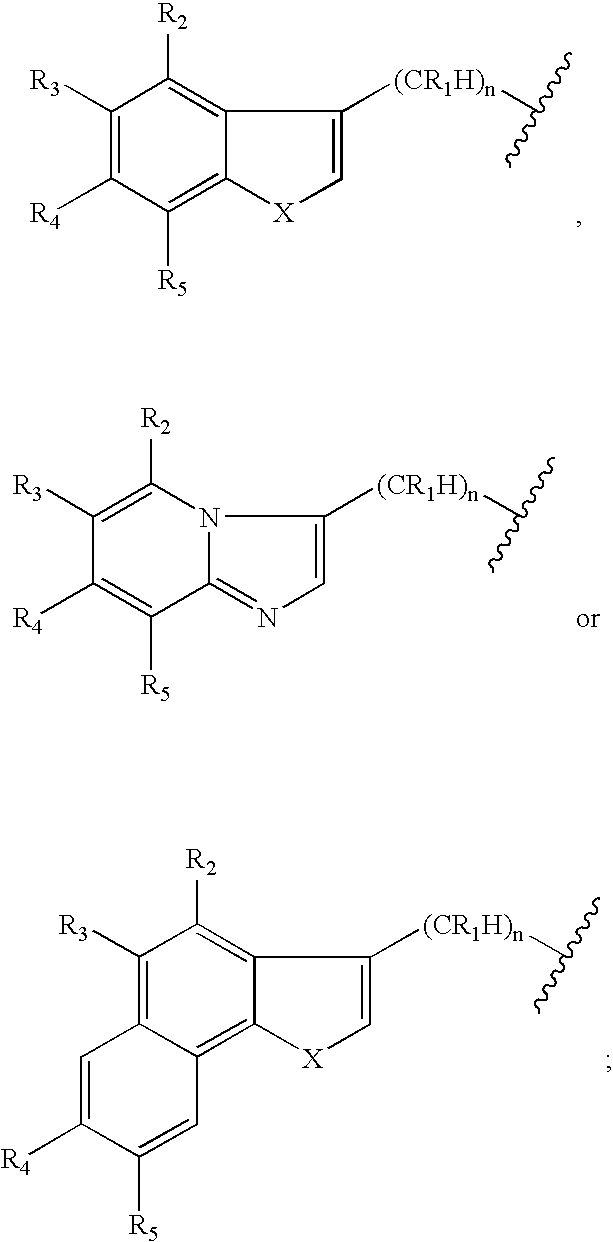
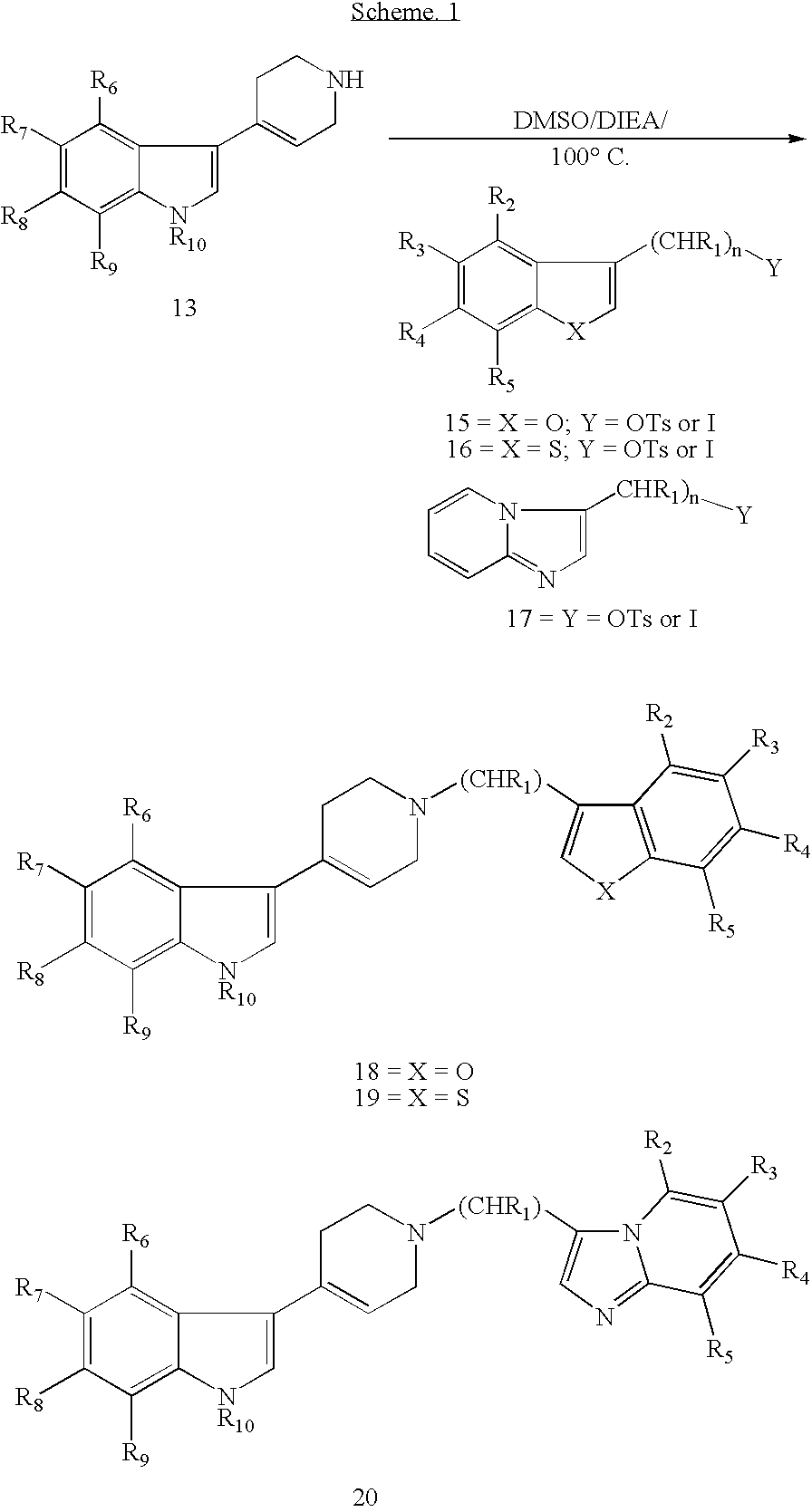
Piperidinyl indole and tetrohydropyridinyl indole derivatives and methods of their use
a technology of tetrohydropyridinyl indole and indole, which is applied in the field of 3piperidin4yl1hindole and 3(1, 2, 3, 6tetrahydropyridin4yl)1hindole derivatives, can solve the problems of robbery of energy or motivation, lack of interest or pleasure, feelings of sadness or emptiness, etc., and achieve the effect of inhibiting the reuptak
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Preparation of 3-{1-[2-(1-benzofuran-3-yl)ethyl]-1,2,3,6-tetrahydro-4-pyridinyl}-1H-indole (“Compound 1”)
[0149] Step 1: To a stirred solution of methyl salicylate (15.2 g, 0.1 mol) and anhydrous potassium carbonate (50.0 g, excess) in acetone (500 ml), ethyl bromoacetate (16.7 g, 0.1 mol) was added. The reaction mixture was refluxed for 24 hrs and cooled to room temperature. It was filtered and concentrated. The oily residue was extracted with chloroform and washed well with water. The organic layer was dried over anhydrous MgSO4 and filtered. It was concentrated and taken to next step without any purification. White oil; Yield: 22.0 g (92%); 239 (M+H).
[0150] Step 2: The methyl-2-(ethoxy-2-oxoethoxy)benzoate obtained from step 1, (11.9 g, 50 mmol) was dissolved in THF:MeOH (1:1) (300 ml) and 5N NaOH (100 ml) was added. The reaction mixture was refluxed for 24 hrs and cooled to room temperature. At the end, it was concentrated to dryness and dissolved in water. The aqueous layer wa...
example 2
Preparation of 3-{1-[2-(1-benzofuran-3-yl)ethyl]-1,2,3,6-tetrahydro-4-pyridinyl}-1-methyl-1H-indole (“Compound 2”)
[0156] 3-{1-[2-(1-benzofuran-3-yl)ethyl]-1,2,3,6-tetrahydro-4-pyridinyl}-1H-indole (342 mg,1 mmol) (obtained from example 1, step 6) in dry THF (50 ml) was slowly added to a stirred suspension of hexane and washed with 60% sodium hydride (44 mg) at 0° C. After the addition, the reaction mixture was stirred for 30 min and methyl iodide (213 mg, 1.5 mmol) was added. The reaction mixture was stirred for 4 hrs and quenched carefully with ice cold water. The reaction mixture was extracted with chloroform; washed well with water; dried over anhydrous MgSO4; filtered and concentrated. The residue obtained was purified by silica-gel column chromatography by eluting it with 50% ethyl acetate:hexane. Light green semi-solid. Yield: 280 mg (78%); 357 (M+H); 1H NMR: δ7.94 (d, 1H), 7.69 (d, 1H), 7.5-7.0 (m, 8H), 6.2 (bs, 1H), 3.8 (s, 3H), 3.49 (bs, 2H), 3.0 (m, 2H), 2.85 (m, 4H), 2.6...
example 3
Preparation of 3-{1-[2-(1-benzofuran-3-yl)ethyl]-1,2,3,6-tetrahydro-4-pyridinyl}-7-ethyl-1H-indole (“Compound 3”)
[0157] 3-{1-[2-(1-benzofuran-3-yl)ethyl]-1,2,3,6-tetrahydro-4-pyridinyl}-7-ethyl-1H-indole was prepared by generally following the procedure outlined in example 1, step 6, starting from the tosylate (316 mg, 1 mmol) and 3 (1,2,3,6-tetrahydro-4-pyridinyl)-7-ethyl-1H-indole (226 mg, 1 mmol). The product was purified by silica-gel column chromatography by eluting it with 70% ethyl acetate:hexane. Brown oil; Yield: 210 mg (56%); 371 (M+H); 1HNMR (400 MHz, CDCl3): δ 8.05 (broad s, 1H, NH); 7.77˜6.90 (m, 9H); 6.20 (s,1H); 3.36˜2.60 (m, 12 H); 1.39˜1.35 (t, J=7.6 Hz, 3H).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com