Antibody variants
a technology of antibodies and variants, applied in the field of antibodies variants, can solve the problem of uneven distribution of variable domains of antibodies, and achieve the effect of reducing the number of antibodies
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
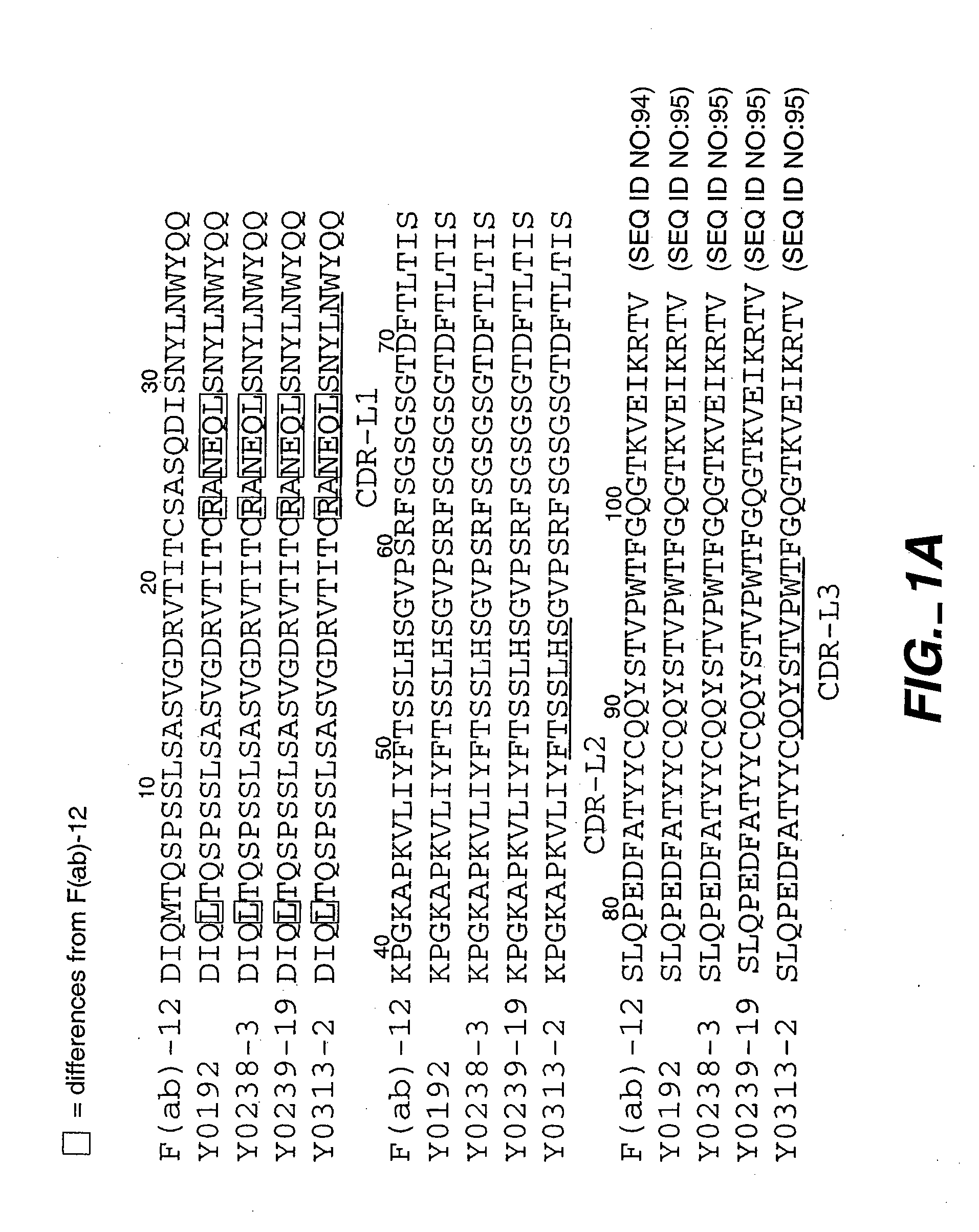
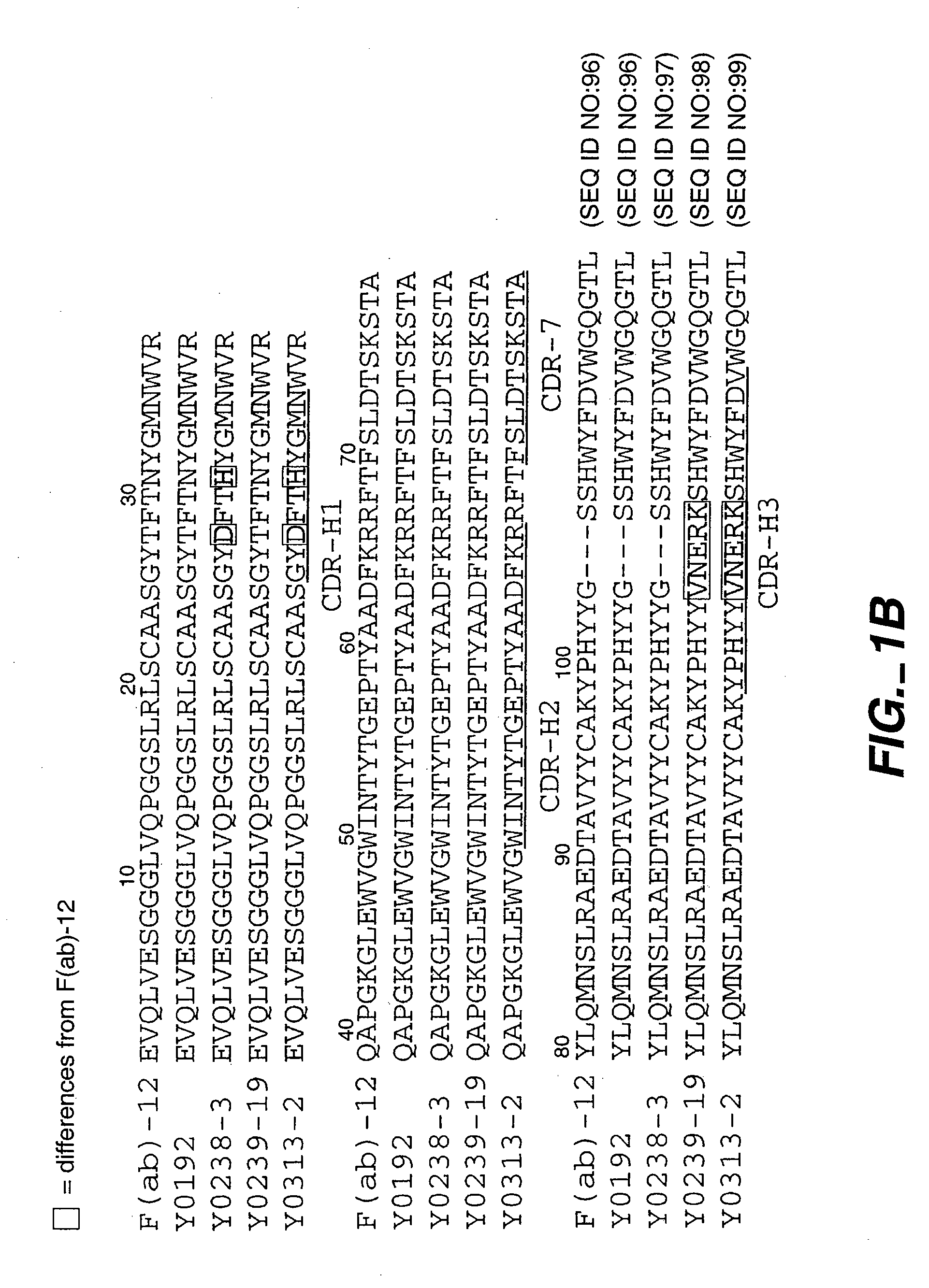
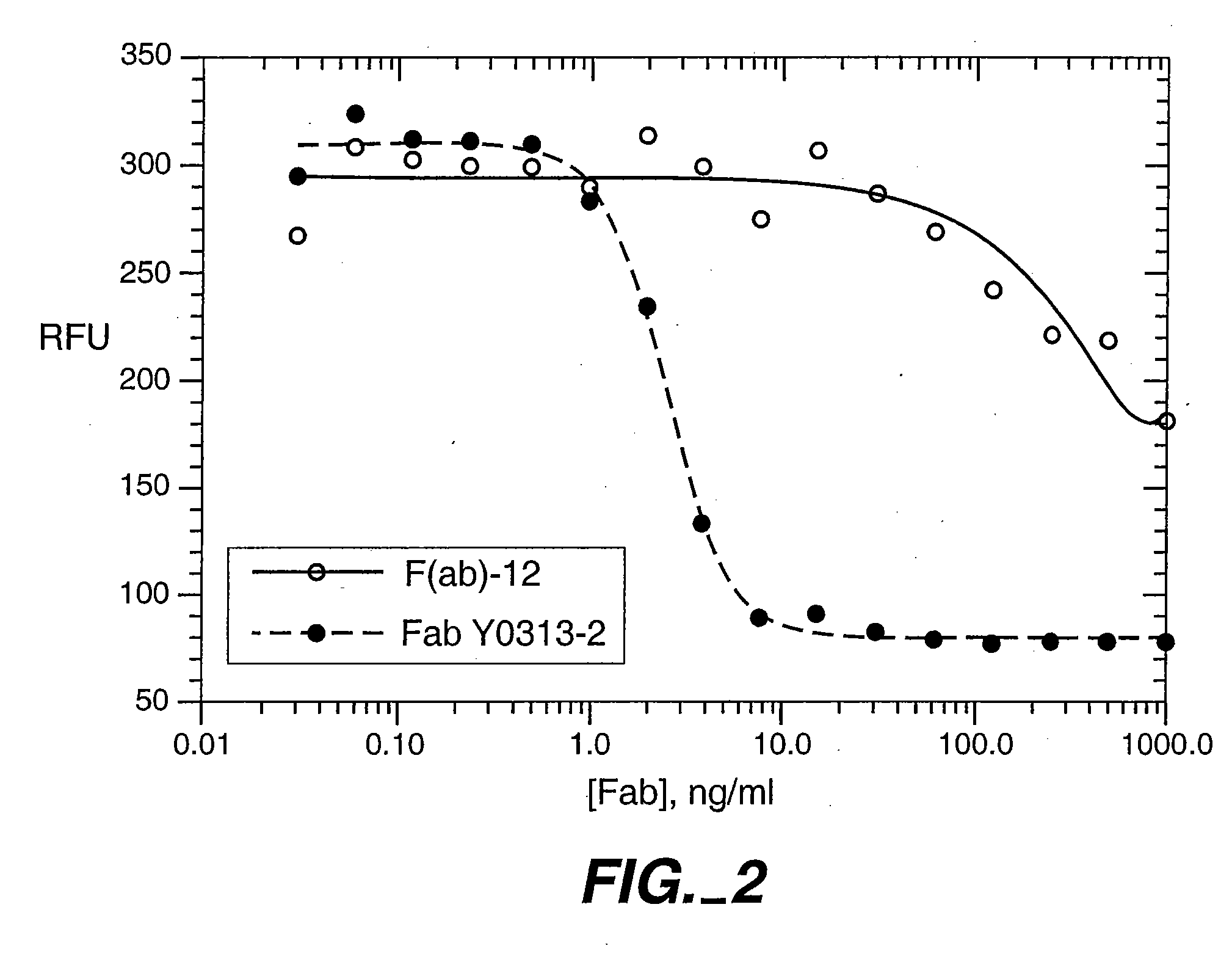
[0237] In this example, antibody variants containing randomized peptide inserts within the antibody CDRs are prepared by phage display which substantially improve the affinity of a humanized Fab for VEGF. Crystallography suggests that these changes result in an increased contact area with antigen.
[0238] VEGF:Fab X-ray Co-Crystal Structure: A crystal structure of the complex between the VEGF antigen and anti-VEGF parent antibody was prepared as described in Muller et al., Structure 6(9):1153-1167 (1998). The conclusion that the three VH CDRs are the main determinants of Fab binding to VEGF is supported by the high-resolution crystal structure of the VEGF:Fab (v36) complex. In addition, the major energetic determinants largely coincide with the principal contacting residues of the Fab in the complex.
[0239] Several randomized libraries were designed with a peptide insertion placed in the antigen-contacting CDRs which, from the crystal structure, were expected to increase the potentia...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Temperature | aaaaa | aaaaa |
| Molar density | aaaaa | aaaaa |
| Molar density | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com