Method for in vivo, ex vivo and in vitro repair and regeneration of cartilage and collagen and bone remodeling
a technology applied in the field of in vivo, ex vivo and in vitro repair and regeneration of cartilage and collagen and bone remodeling, can solve the problems of reducing personal productivity and quality of life, increasing morbidity and mortality for men and women, and the need for revision arthroplasty
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Chondrocyte Isolation
[0170]This example describes procedure used for isolation of chondrocytes.
[0171]Adult bovine articular chondrocytes were isolated from full thickness cartilage dissected from radiocarpal joints obtained fresh from a local abattoir.
[0172]The cartilage cells were released from the matrix by incubation in 15 ml of Dulbecco's modified Eagle's medium (DMEM) containing gentamicin (50 ug / ml) and a mixture of bacterial collagenases, type II and type IV, (Worthington, Freehold, N.J.) at a final concentration of 0.6 mg / ml each in 15 ml of Dulbecco's Modified Eagle's Medium / Ham's F12 (DMEM / F12, Gibco BRL, Grand Island, N.Y.) containing 25 μg / ml gentoamicin (Sigma, St. Louis, Mo.). The cartilage samples were incubated for a total of 18 hours at 37° C. in 7.5% CO2 and 100% humidity to ensure complete digestion. Chondrocytes released from matrix were filtered through a nylon mesh filter to isolate single cells. The cells were subsequently collected by repeated centrifugation ...
example 2
Serum Containing or Serum-Free Medium
[0175]This example describes a composition of serum containing or serum-free medium.
[0176]Serum containing Dulbecco's modified Eagle's medium (DMEM) contained dialyzed heat-inactivated fetal bovine serum at a concentration of 10% v / v. Serum-free medium consisted of a 1:1 mixture of Ham's F12 / DMEM supplemented with selenium, and liposomes. Liposomes were prepared by dissolving lecithin, cholesterol, sphingomyelin, and vitamin E acetate in 1 ml of 2:1 chloroform / methanol (vol / vol) which is dried under N2. One ml of DMEM / F12 was added and the lipid mixture was then sonicated 3× for intervals of 3 minutes each, using a microtip with a 70% duty cycle. This liposome stock was made up at 1,000× the final concentration, kept under N2, and stored at 4° C. In some experiments, ascorbate was added to the medium at a concentration of 50 μg / ml.
example 3
Mechanical Loading with Intermittent Hydrostatic Pressures
[0177]This example describes loading protocol and conditions for applying intermittent hydrostatic pressure.
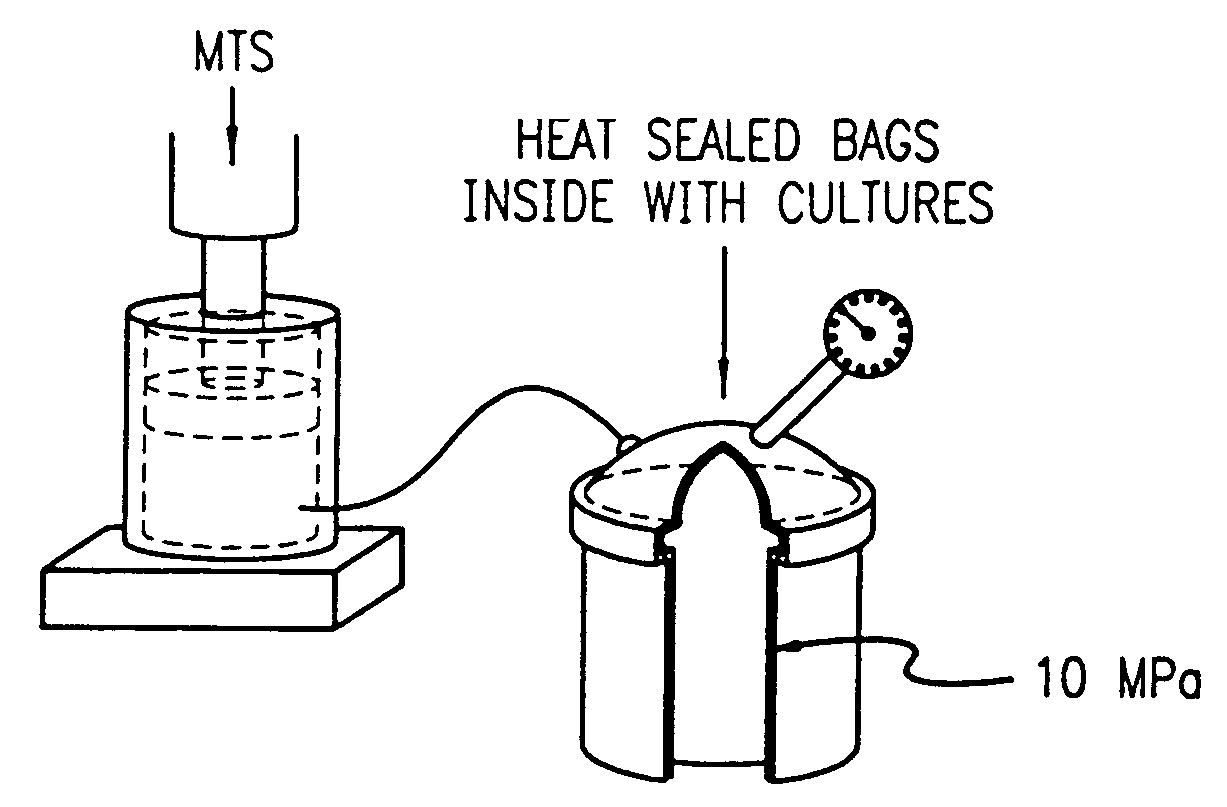
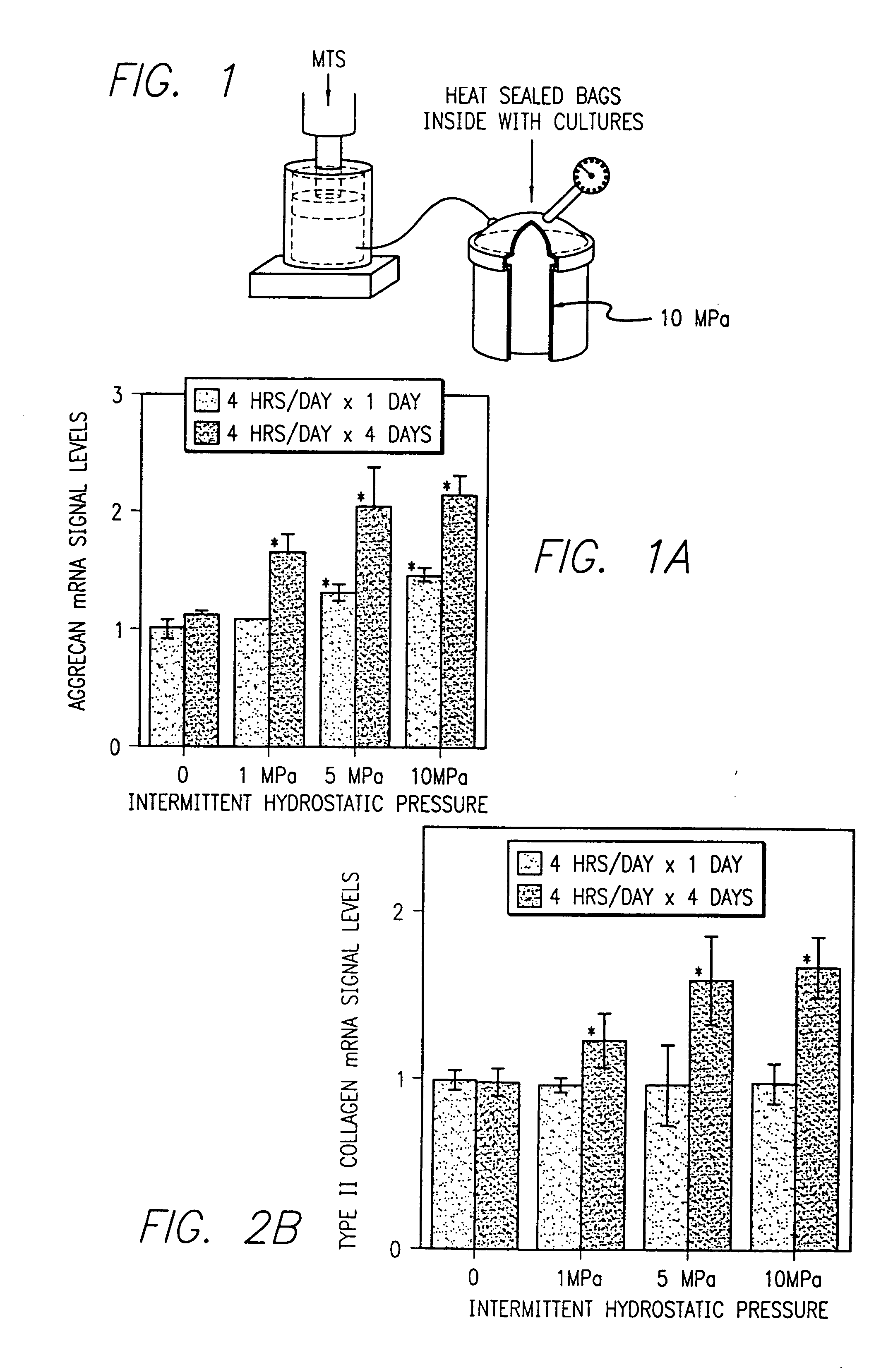
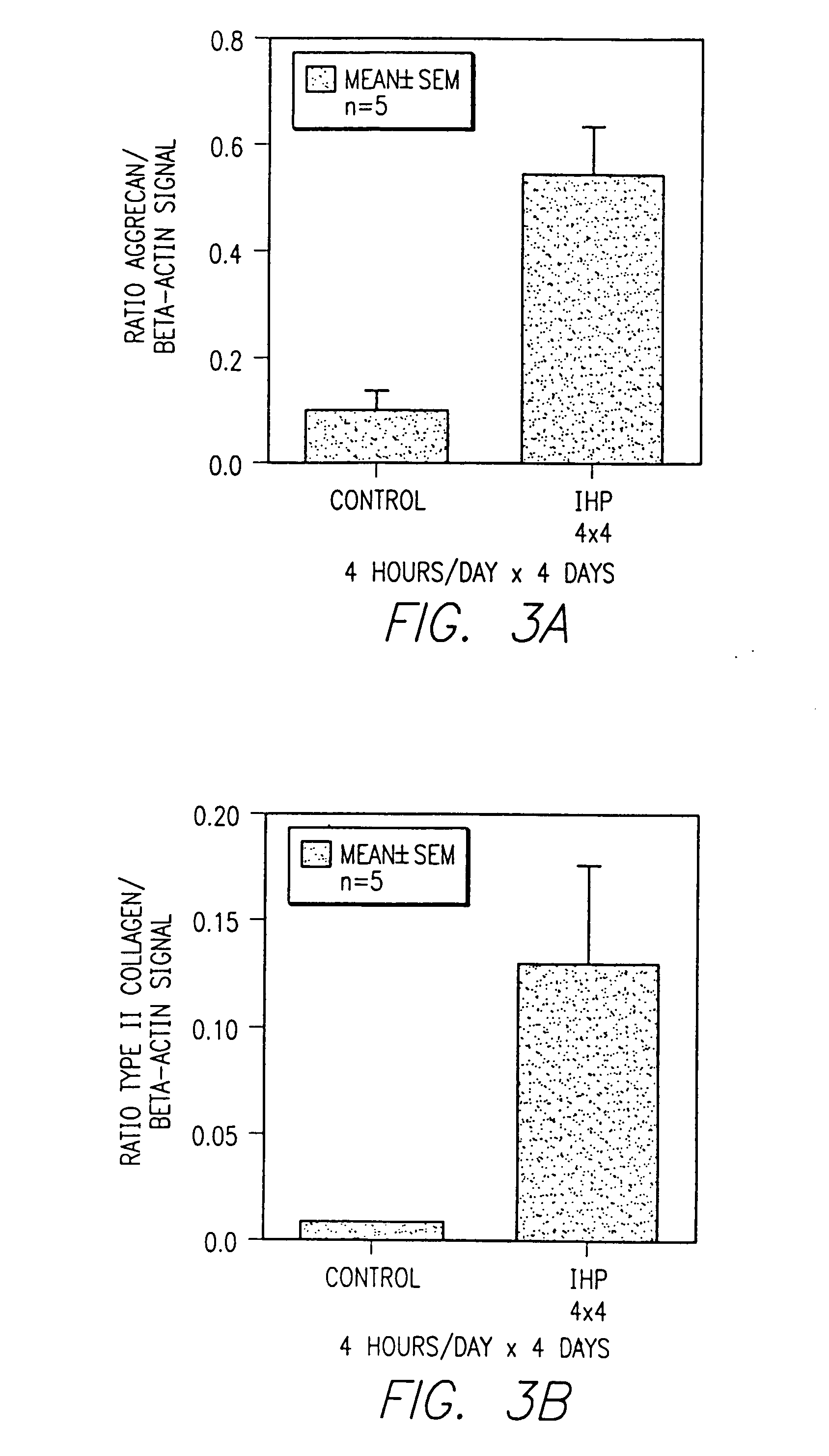
[0178]Hydrostatic pressure was cyclically applied at a loading dose of 10 MPA and at a frequency of 1 Hz. The intermittent hydrostatic pressure was applied continuously with cells removed at periods of 2, 4, 8, 12 and 24 hours, or through an interval loading protocol with the cells removed after a 4 day period during which intermittent hydrostatic pressure was applied for and limited to 4 hours per day followed by 20 hours of recover. This was repeated daily for four days or more. Each experimental time point was tested in triplicate and each experiment was carried out for a minimum of three independent trials.
[0179]The pressure was generated with a commercially available stainless steel pressure vessel interfaced to a servo-hydraulic loading instrument seen in FIG. 1. The design provided for the complete evacuation of ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| frequency | aaaaa | aaaaa |
| hydrostatic pressure | aaaaa | aaaaa |
| hydrostatic pressure | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com