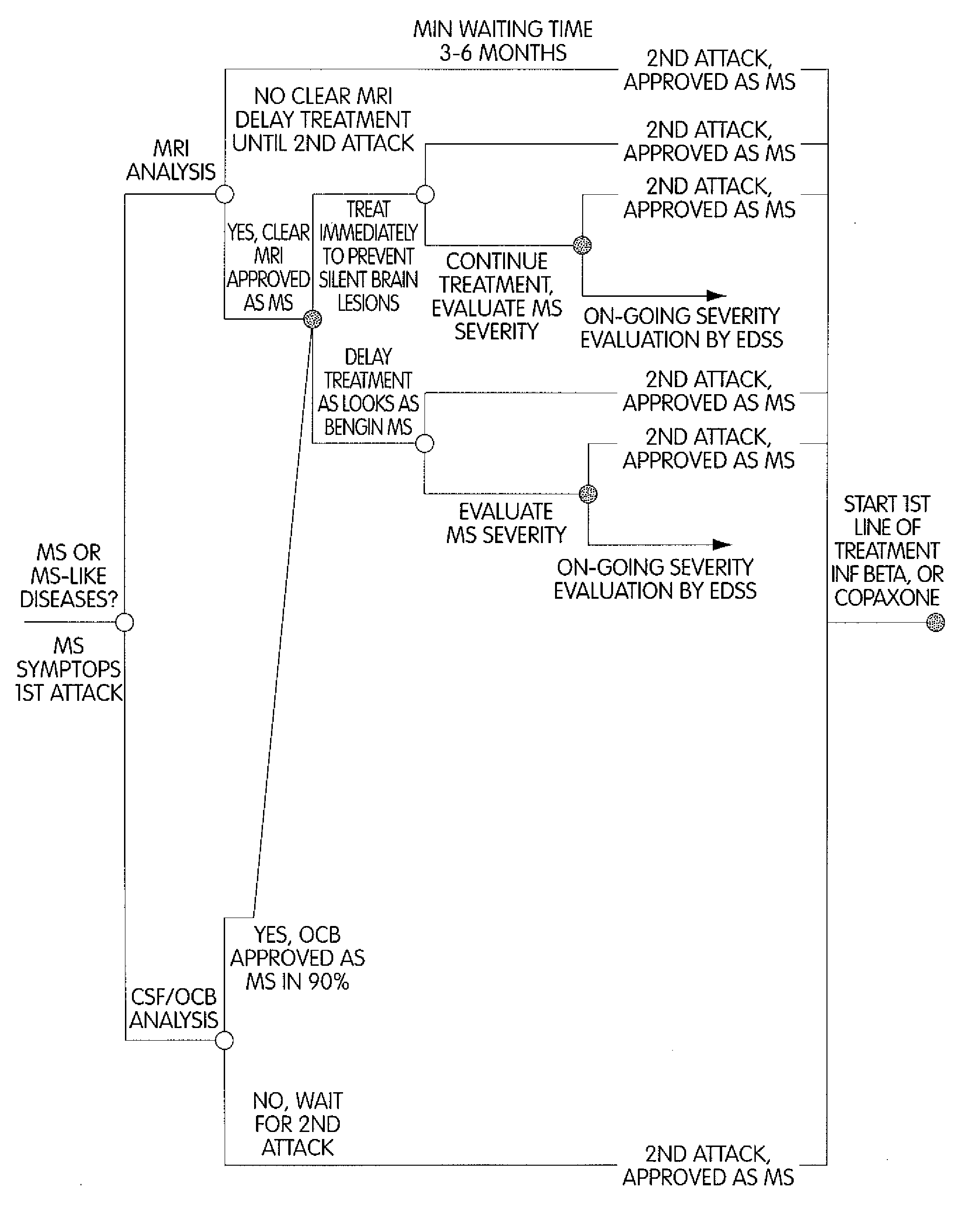
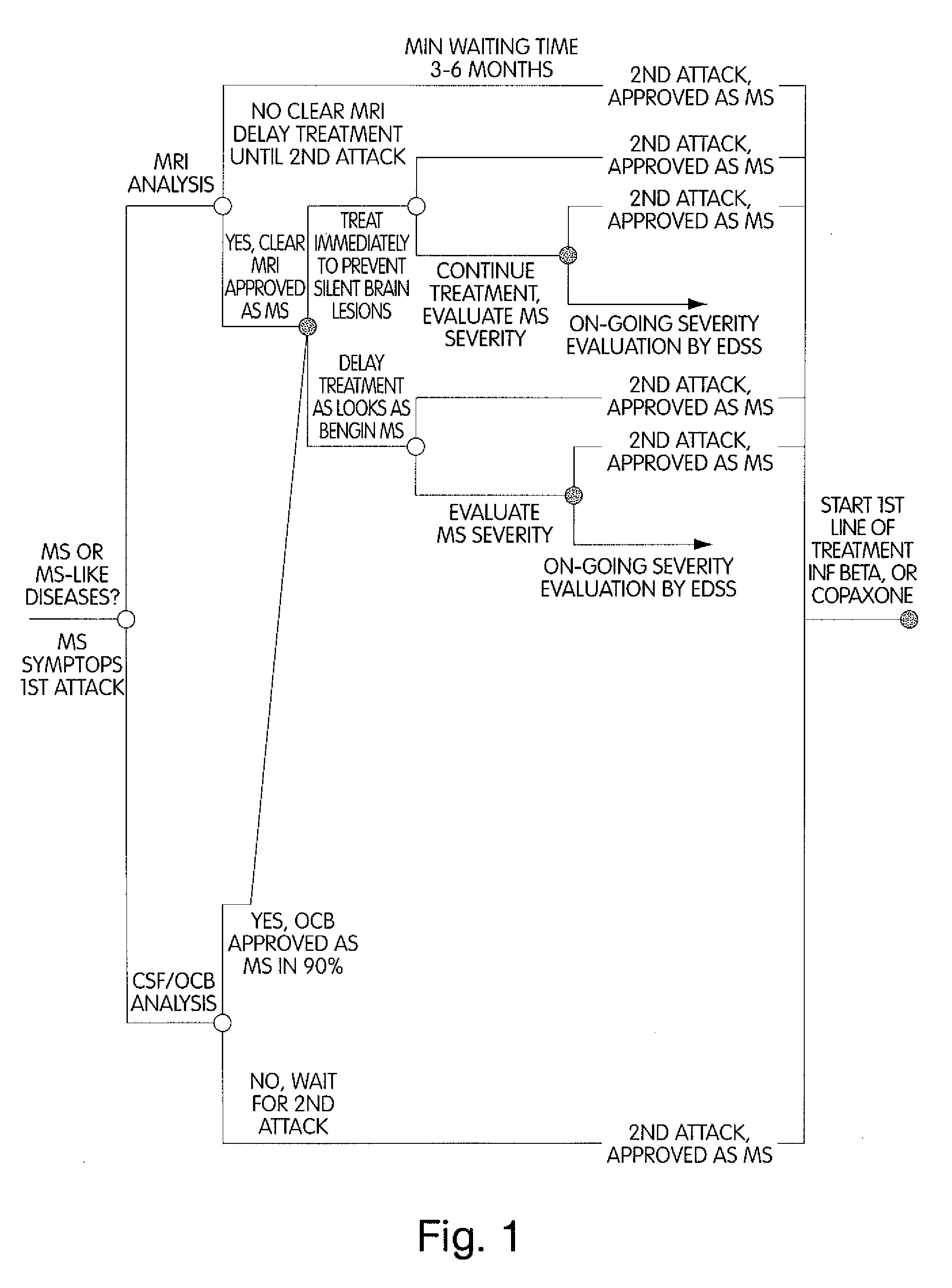
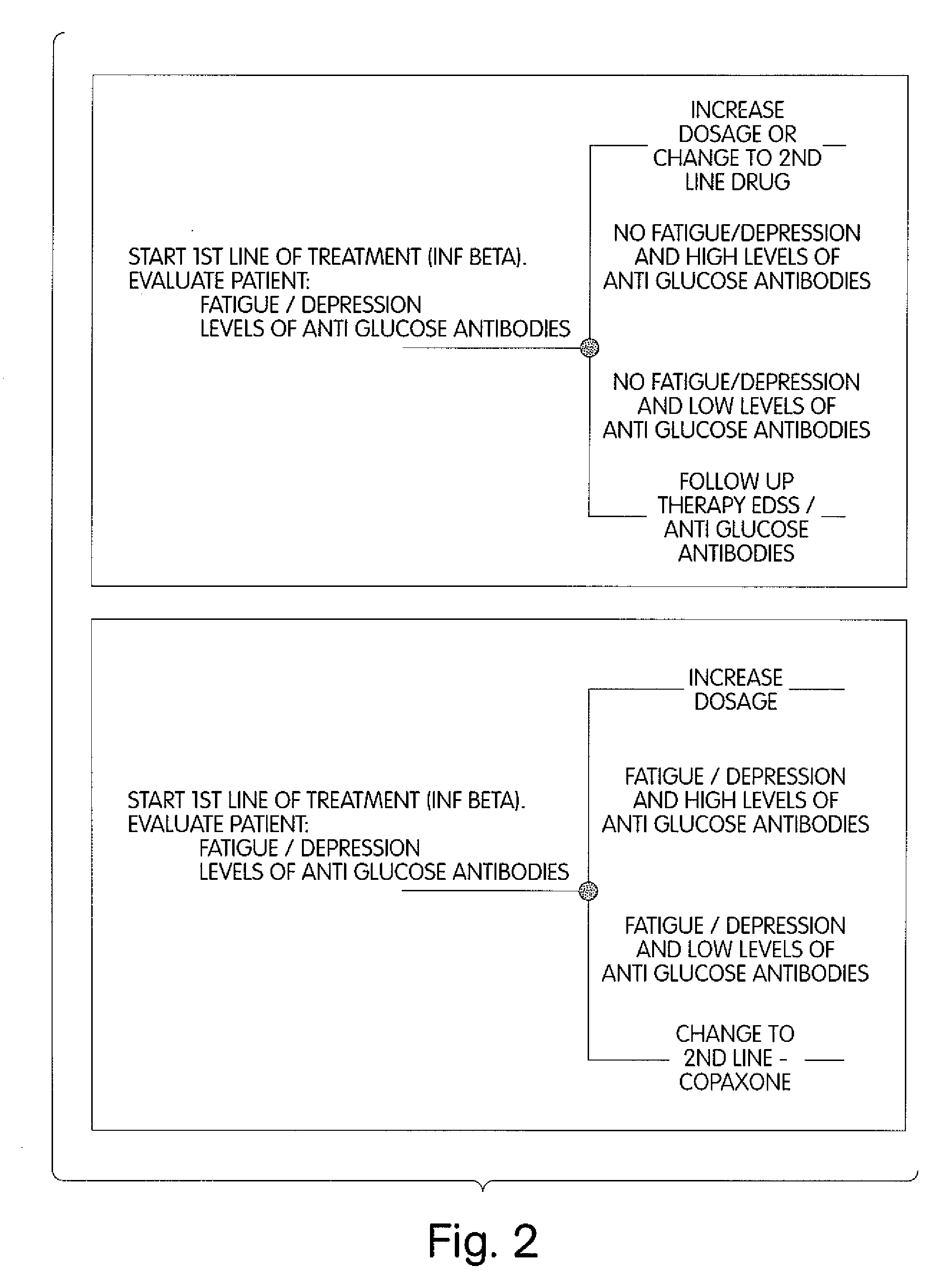
Method for Diagnosing Multiple Sclerosis
a multiple sclerosis and multiple sclerosis technology, applied in the field of multiple sclerosis diagnosis, can solve the problems of poor correlation between mri results and disease activity, persistent disability in young adults, and high cost of routine use of mri, and achieve the effect of elevating serum levels
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Comparison Between Antiglycan Antibodies in the Serum of Multiple Sclerosis (MS) Patients and Normal Population
[0078]An anti-glycan antibody (Igs) profile was obtained using GlycoChip® arrays (Glycominds, Ltd., Lod, Israel, Cat No. 9100). The arrays were constructed using procedures described in WO00 / 49412. Anti-glycan antibody profiles of 40 multiple sclerosis patients and 40 sex and aged matched normal blood donors were compared.
[0079]All serum samples were tested using GlycoChip® plates (Glycominds Ltd., Lod, Israel, Cat No. 9100), which was an array of mono and oligosaccharide covalently attached to a reduced volume 384 wells micro titer plate. The mono and oligosaccharides displayed on the array are listed in FIG. 4. A translation of the LinearCode™ syntax used to describe glycan structure to IUPAC nomenclature can be found in Table 1.
[0080]The sera of healthy volunteers and MS patients volunteers who had signed an informed consent form were collected in evacuated silicon coate...
example 2
Differences in the Levels of Anti-Glc (α), and Anti-Glc (α 1-4) Glc (α), a IgM Antibodies in the Serum Between Ms Patients in Attack, Stable Ms Patients and Healthy Population
[0087]A glycan array was used to search for biomarkers among the human serum glycan binding antibody repertoire to differentiate between a healthy population and a group of Multiple Sclerosis (MS) patients, and between MS patients in exacerbation and remission stages. This example demonstrates that two IgM antibodies, anti-Glc (α) and anti-Glc (α 1-4) Glc (α), are found at significantly higher levels in MS patients than in healthy people (sensitivity and specificity of 60% and 93%, respectively), and in MS patients in an exacerbation stage relative to patients in a remission stage (sensitivity and specificity of 89% and 71%, respectively). Also provided is an anti-glycan antibody profile for a healthy population, including a range of variation during a 13 week interval.
[0088]The temporal stability of antiglycan...
example 4
Anti-Glycan Antibody Profile (AGAP) in a Normal Human Population
[0096]Total Ig antibody binding (as detected with Protein A) of 72 individual sera to 34 mono- and oligosaccharides (FIG. 11 and Table 5), and IgG, IgA, and IgM binding of 200 sera to six mono- and oligosaccharides (FIG. 15A-C) was determined. The strongest signals were recorded for antibodies against GlcNAc (α) and L-Rha (α), while lower levels were observed against β4-linked oligosaccharides of glucose, GlcNAc (β), GlcNAC (β 1-4) GlcNAC (β), Gal (α) and Gal (a 1-3) Gal (β 1-4) GlcNAc (β). This is in good agreement with previously published data showing the distribution of anti-glycan antibodies in a commercially available human serum pool (WO02 / 064556). The AGAP of subclasses IgG and IgA were similar to the total Ig AGAP, while that of IgM was lower and more uniform among the different glycans. The anti-glycan antibodies of the population tended to fit a lognormal distribution see FIG. 15A-15C. It is evident that cons...
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentrations | aaaaa | aaaaa |
| pH | aaaaa | aaaaa |
| volume | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com