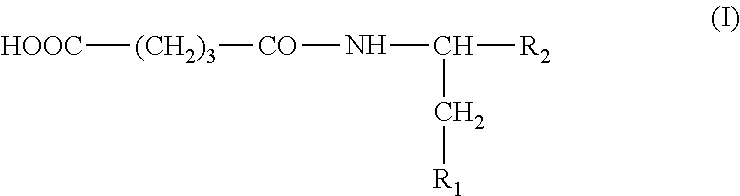
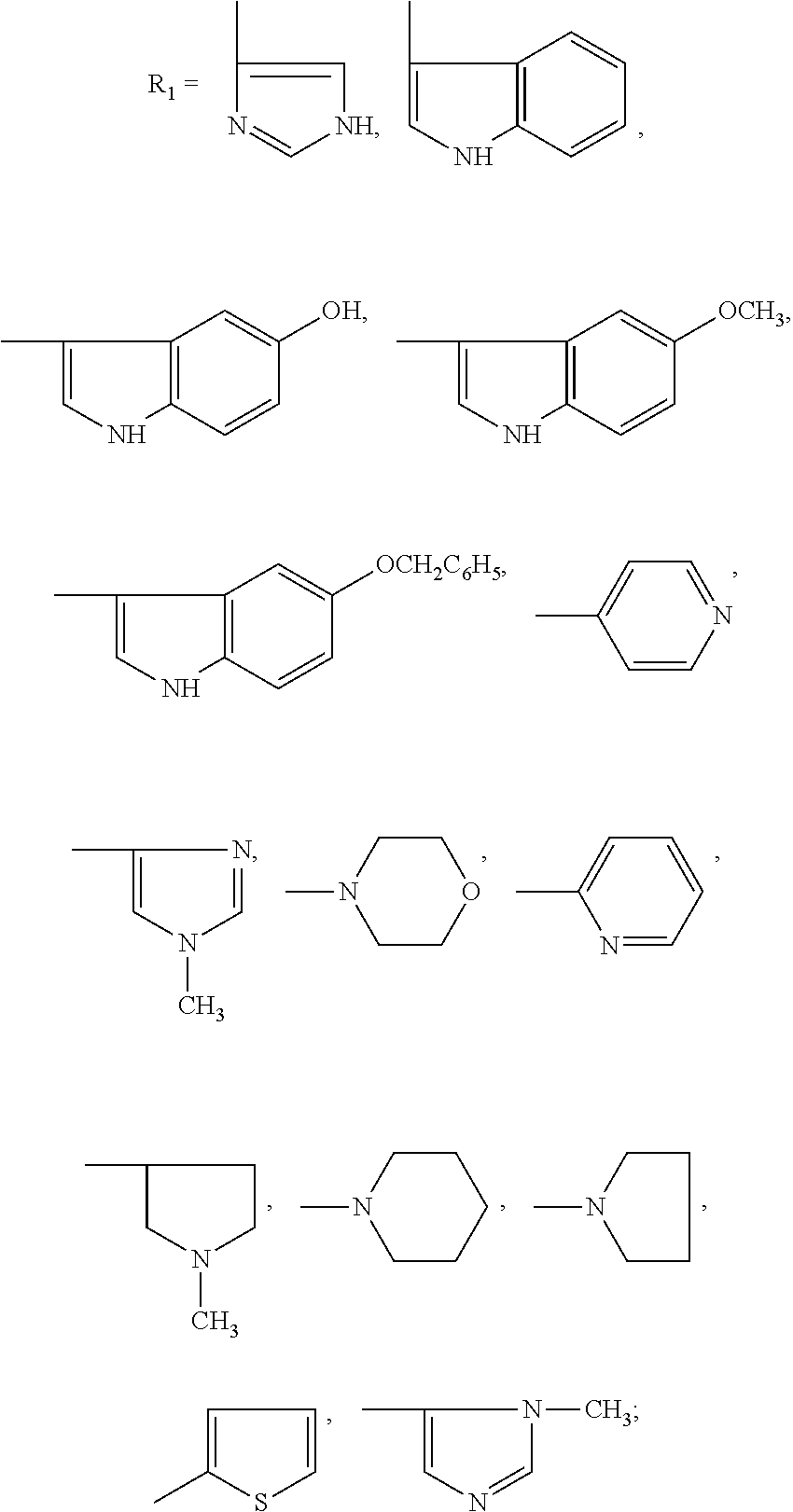
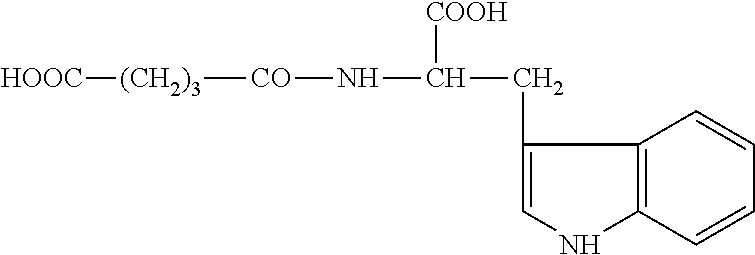
Use of Glutaric Acid Derivatives or the Pharmaceutically Acceptable Salts Thereof as Anti-Arrhythmic Agents
a technology of glutaric acid and derivatives, which is applied in the direction of biocide, plant growth regulators, animal husbandry, etc., can solve the problems of low anti-arrhythmic activity upon parenteral administration, low therapeutic diapason, and insufficient effectiveness
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
Anti-Arrhythmic Activity of Compounds of General Formula (I) and N-succinyl-D,L-tryptophane Dipotasassium Salt and Also the Effect Thereof on Mortality Upon Adrenal Arrhythmia in Mice
[0034]Adrenal disorders of cardiac rhythm were reproduced on pedigreeless white laboratory mice of either sex having a weight of 18-22 g. Experimental arrhythmia in the animals was caused in accordance with the method described in the source Moore E. N., Spear J. F. Acute animal models for the study of antiarrhythmic drugs for the prevention of sudden coronary death. / / Clin. Pharmacol. Antiarhythmic Therapy. New-York. 1984. pp. 31-46.
[0035]The results of comparative testing of the claimed compounds and N-succinyl-D,L-tryptophane dipotasassium salt are presented in Table 2.
TABLE 2Effectiveness of compounds of general formula (I) and N-succinyl-D,L-tryptophane dipotasassium salt upon adrenal intoxication in miceNumber of animals in groupswithTrial conditions, doseventricularwith AVLifetimeNo.(mg / kg)in tri...
example 2
Activity of Compound III (Nα-glutaryl-L-histidine) and of N-succinyl-D,L-tryptophane Dipotassium Salt on Models of Aconitic Arrhythmia in Mice
[0039]Arrhythmia was reproduced according to the method of Ju. I. Vikhlyayev and N. V. Kaverina (1958) [Kaverina N. V., Berdyaev S. Ju., Kuschuk E. P., Paskhina O. E. Methodical indications in respect to a study of the anti-arrhythmic activity of new pharmacological substances. Manual on experimental (pre-clinical) study of new pharmacological substances. Edited by V. P. Fisenko.—Moscow. 2000. Page 210]. Aconitine nitrate was administered to the animals in a dose of 50 μg / kg intravenously.
[0040]This model makes it possible to assess the scope of the therapeutic action of the presented compounds and the N-succinyl-D,L-tryptophane dipotassium salt. Assessment of the scope of the therapeutic action was carried out in accordance with the value of the anti-arrhythmic index (AAI), determined as the ratio LD50 upon the oral method of administering to...
example 3
A Study of the Effect of Compound III (Nα-glutaryl-L-histidine) and of N-succinyl-D,L-tryptophane Dipotassium Salt on the Course of Early Occlusive Arrhythmia in Cats
[0042]At present, in order to assess the effectiveness of therapy under conditions of transistor ischemic arrhythmogenesis, a method is used that is described by Storozhuk V G. [Antifibrilic activity of some anti-arrhythmic agents upon maximum coronary artery ligation and the reperfusion thereof in cats. Pharmacology and Toxicology. 1985. No. 3, pp. 47-49].
[0043]The results of a study of compound III (Nα-glutaryl-L-histidine), N-succinyl-D,L-tryptophane dipotassium salt and classical anti-arrhythmics are presented in Table 4.
TABLE 4Anti-arrhythmic activity of compound III (Nα-glutaryl-L-histidine),comparative preparations and N-succinyl-D,L-tryptophane dipotassiumsalt on models of occlusive arrhythmia in catsStudiedNumber ofTime ofsubstance oranimals withoccurrence ofAnimalspreparation,ventriculararrhythmia,died inNo.do...
PUM
| Property | Measurement | Unit |
|---|---|---|
| weight | aaaaa | aaaaa |
| frequency | aaaaa | aaaaa |
| pH | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com