BTLA Antibodies and Uses Thereof
a technology of antibodies and antibodies, applied in the field of btla antibodies, can solve the problems of not being able to overcome the immunosuppressive mechanisms observed in cancer patients, and no satisfactory approach has been proven to induce potent immune responses against vaccines
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example
Abstract
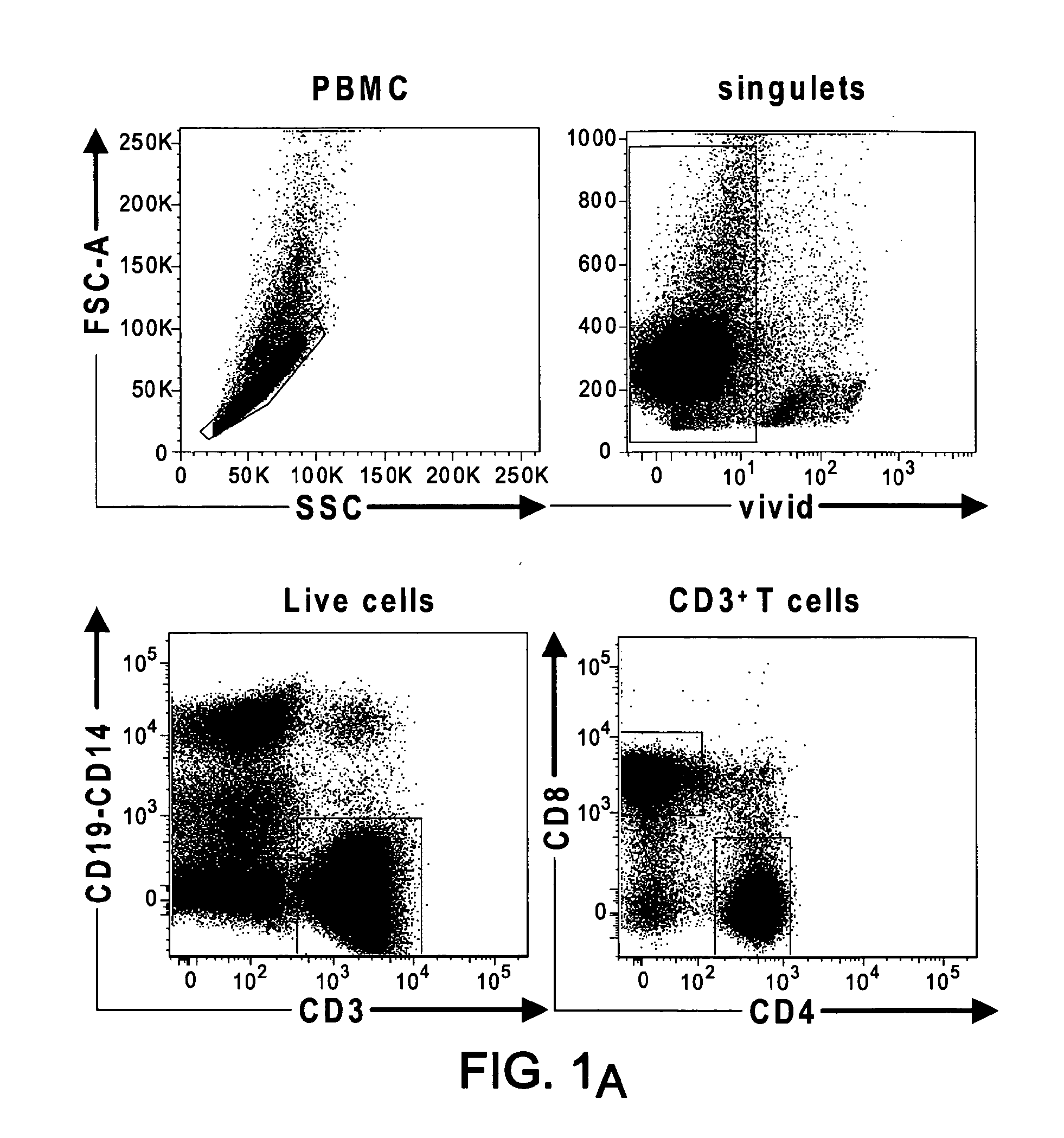
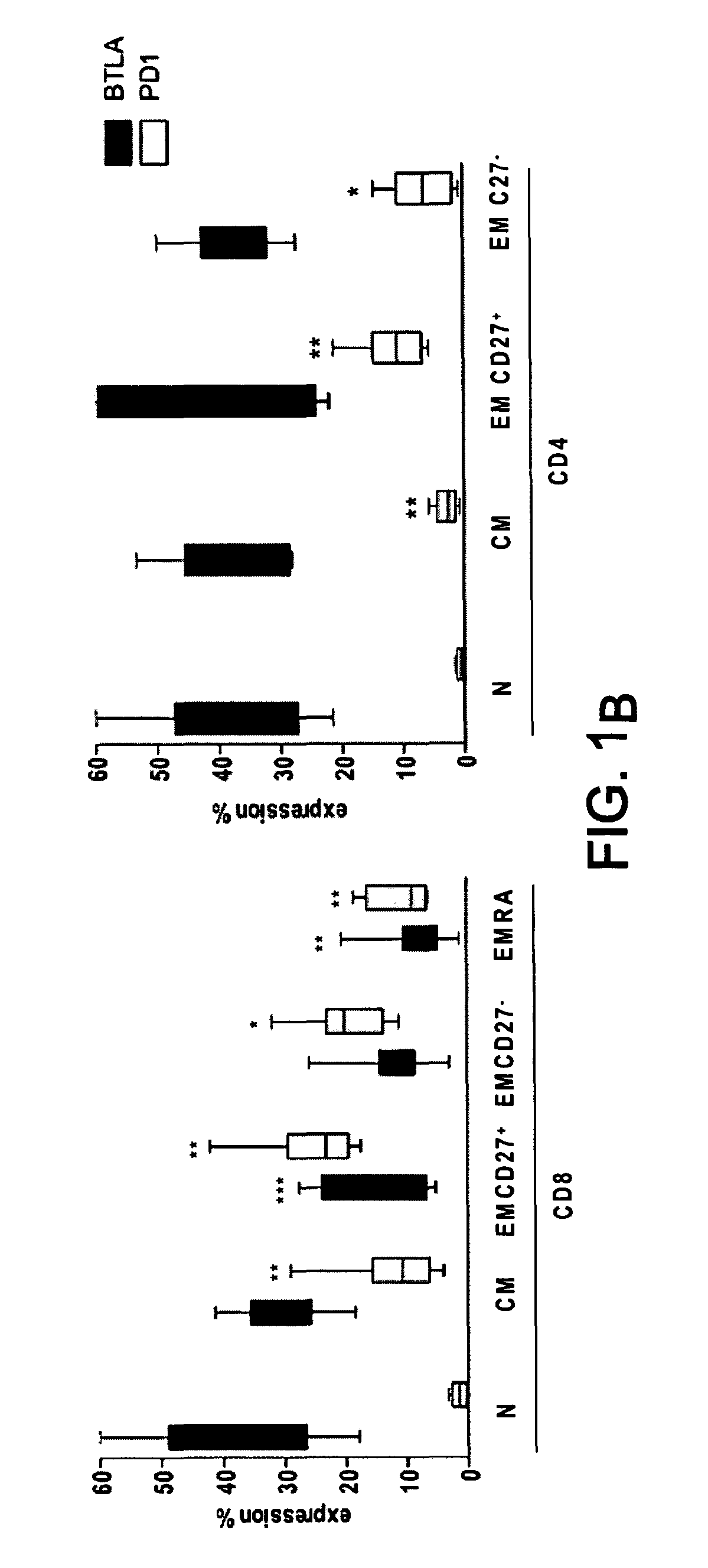
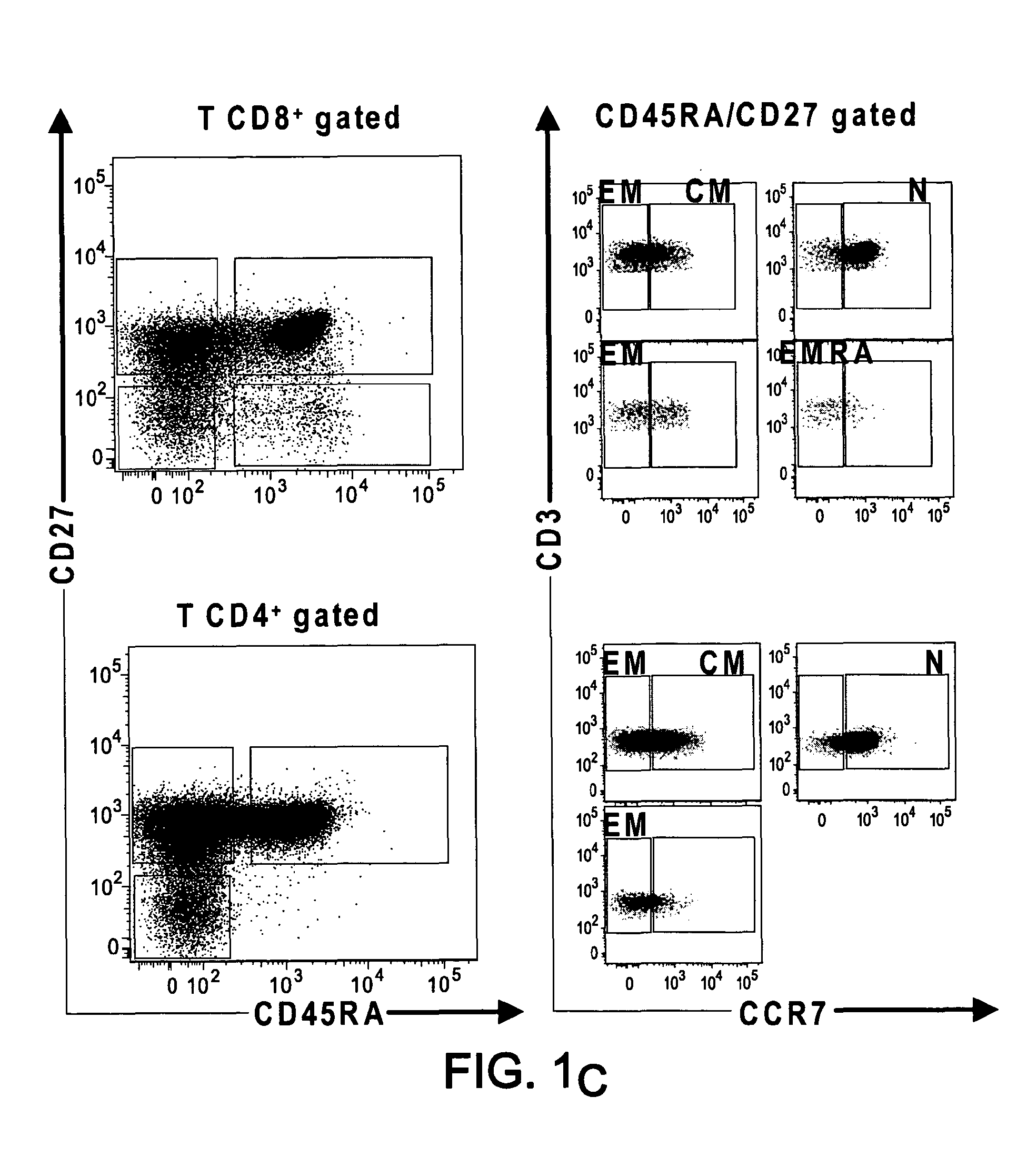
[0148]PD-1 and BTLA are receptors that negatively regulate T-cell activation. We have investigated their respective expression on human T cell subsets and their regulation following activation and finally compared their role in the regulation of T cell functions since there is no side by side comparison of their respective distribution and function. BTLA is expressed on naive CD4 and CD8 T cells and its expression was down-regulated on Effector-type and Memory type CD8+ T cells. In contrast, PD-1 was preferentially expressed by Effector and Memory type CD4+ and CD8+ T cells rather than Naive T cells. Engagement of PD-1 and / or BTLA by agonistic specific monoclonal antibodies blocked by the same strength CD3 / CD28-mediated T cell proliferation and Th1 and Th2 cytokine secretion. However, blockade of PD-1 and / or BTLA engagement following allogenic stimulation of T cells by DCs, revealed a robust inhibitory effect of PD-1 as compared to BTLA and no additive effect was detected be...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameters | aaaaa | aaaaa |
| domain structure | aaaaa | aaaaa |
| binding affinity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com