Method for opening tight junctions
a tight junction and opening technology, applied in the direction of drug compositions, antibody medical ingredients, extracellular fluid disorders, etc., can solve the problems of increased infection risk, difficult to give injections on a regular basis, and many patients are reluctant or unable to give themselves injections. , to achieve the effect of facilitating mucosal delivery of biotherapeutic compounds
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
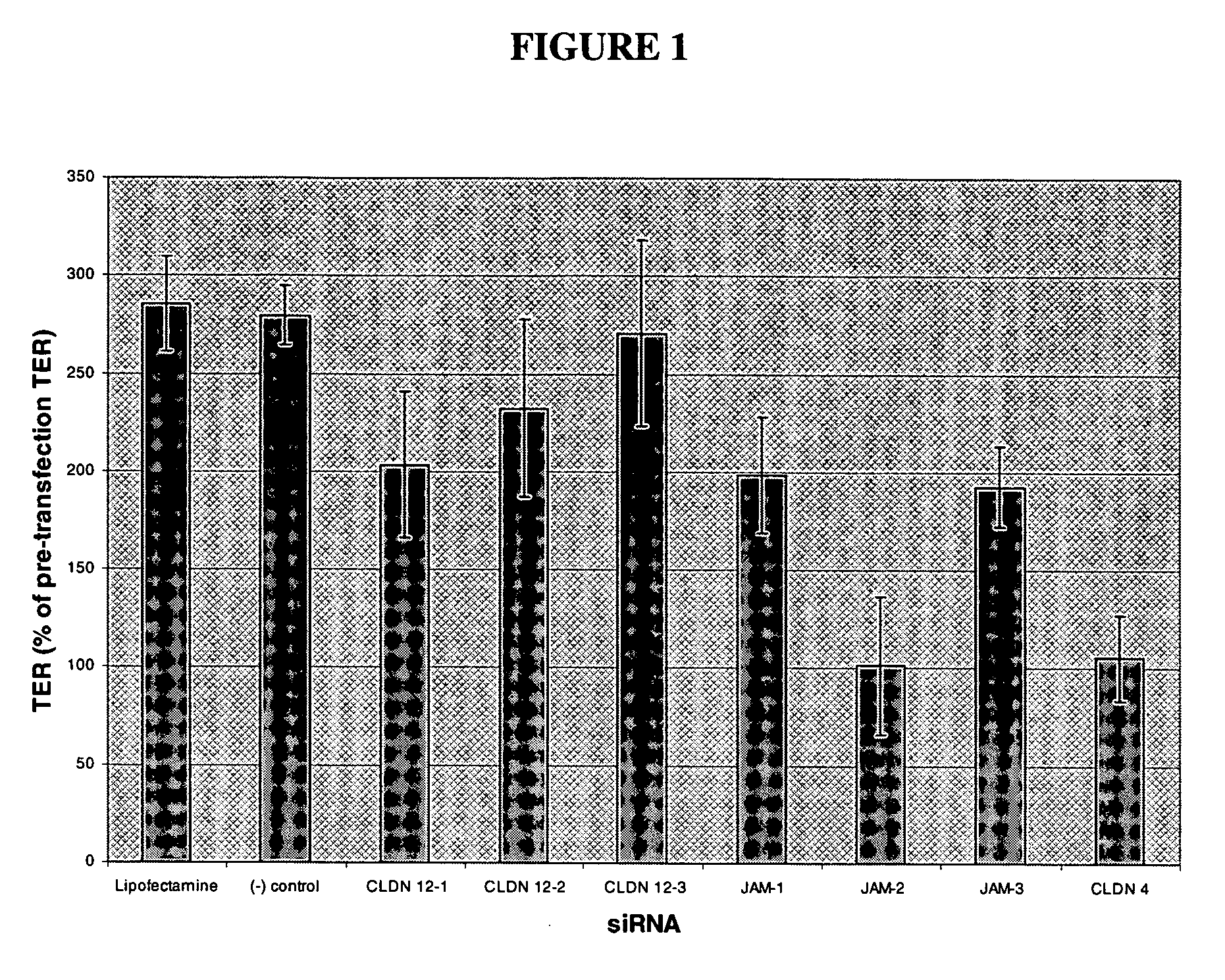
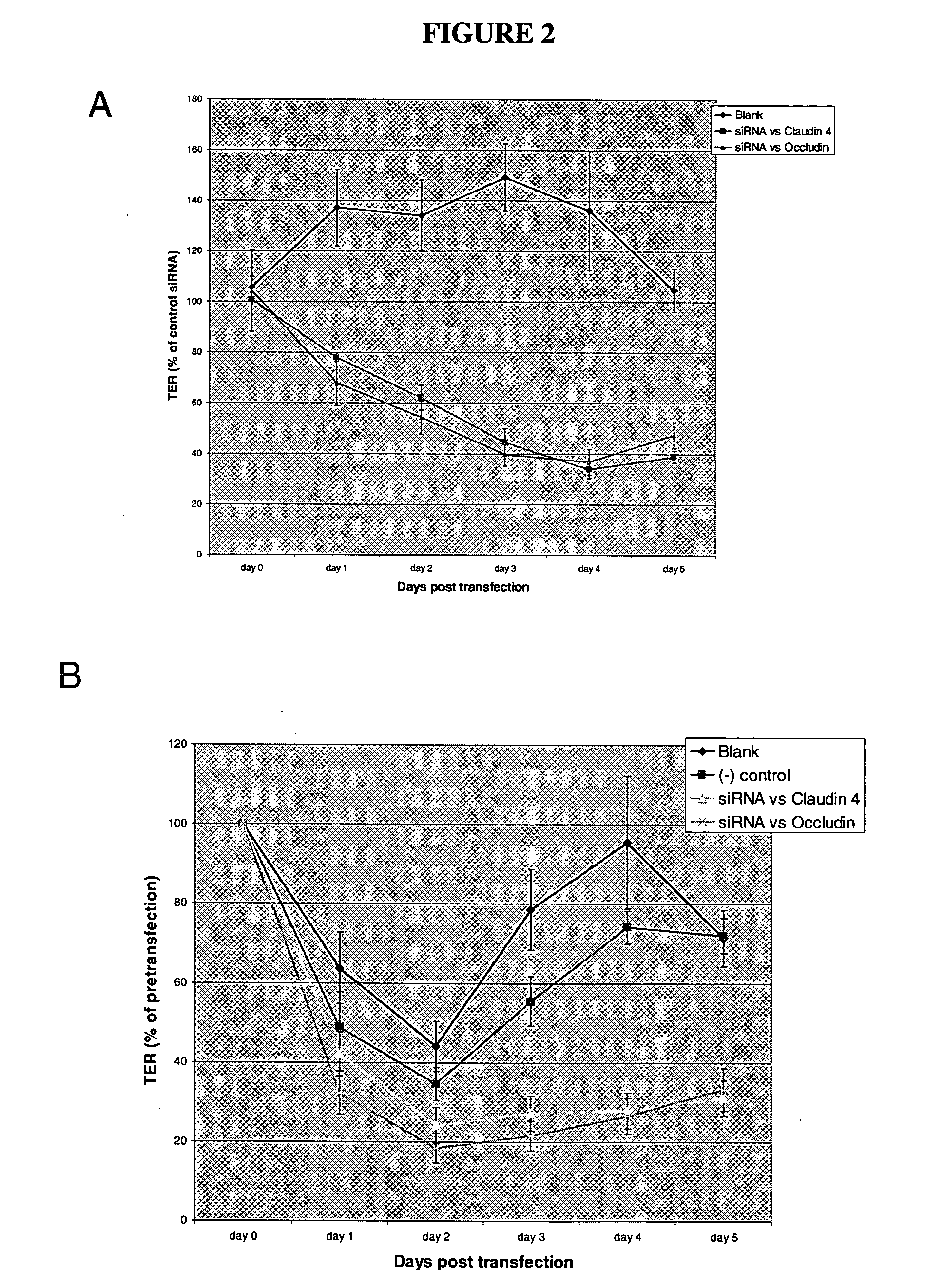
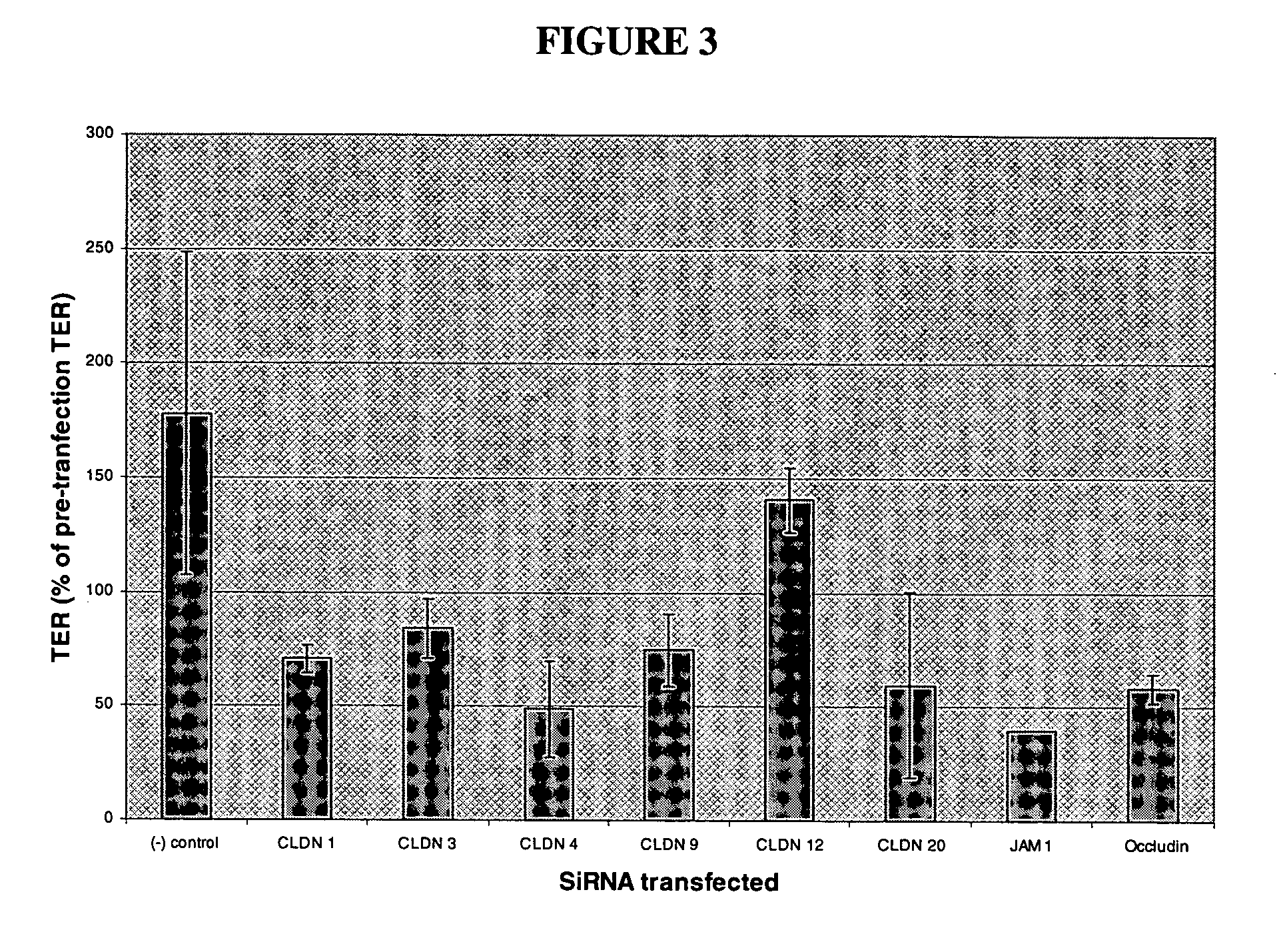
[0106] Tight junctions (TJ) form at the apical end of lateral membranes as closed contacts between the plasma membranes of neighboring cells. The TJ serves as a diffusion barrier between compartments and it is crucial for the development and function of epithelial tissues. Previous studies have suggested that the intra-membrane connections are formed by non-covalently linked branched polymers containing Claudins (CLDNs), Occludin and Junctional Adhesion Molecules (JAMs). These transmembrane proteins interact with multiple components of a cytoplasmic plaque consisting of different types of cytosolic proteins that interact with an actin-based cytoskeleton. There are at least 20 different claudins in epithelial tissues. CLDN tissue-specific expression has been demonstrated in normal as well as in malignant epithelial tissues.
[0107] Studies of the structure and functional properties of the TJ will not only provide insight on how the barrier is formed, but will identify those components...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
| pore size | aaaaa | aaaaa |
| speed | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com