Method and apparatus for delivering an implant without bias to a left atrial appendage
a left atrial appendage and left atrial technology, applied in the field of left atrial appendage delivery without bias, can solve the problems of irregular and turbulent blood flow in the vascular system, rapid and chaotic heartbeat, and failure to contract with any vigor, so as to reduce, eliminate, and/or eliminate the effect of implantation bias
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
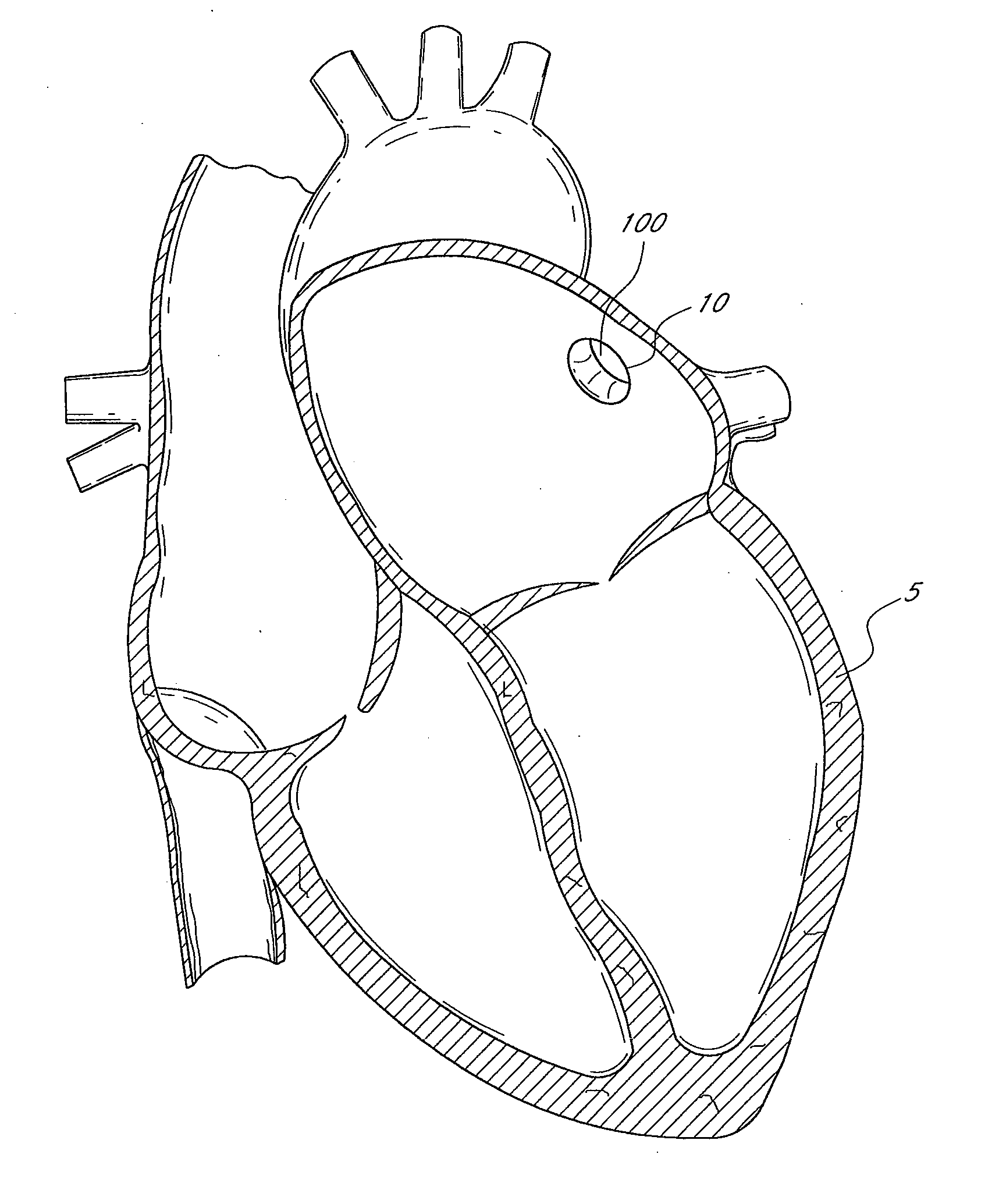
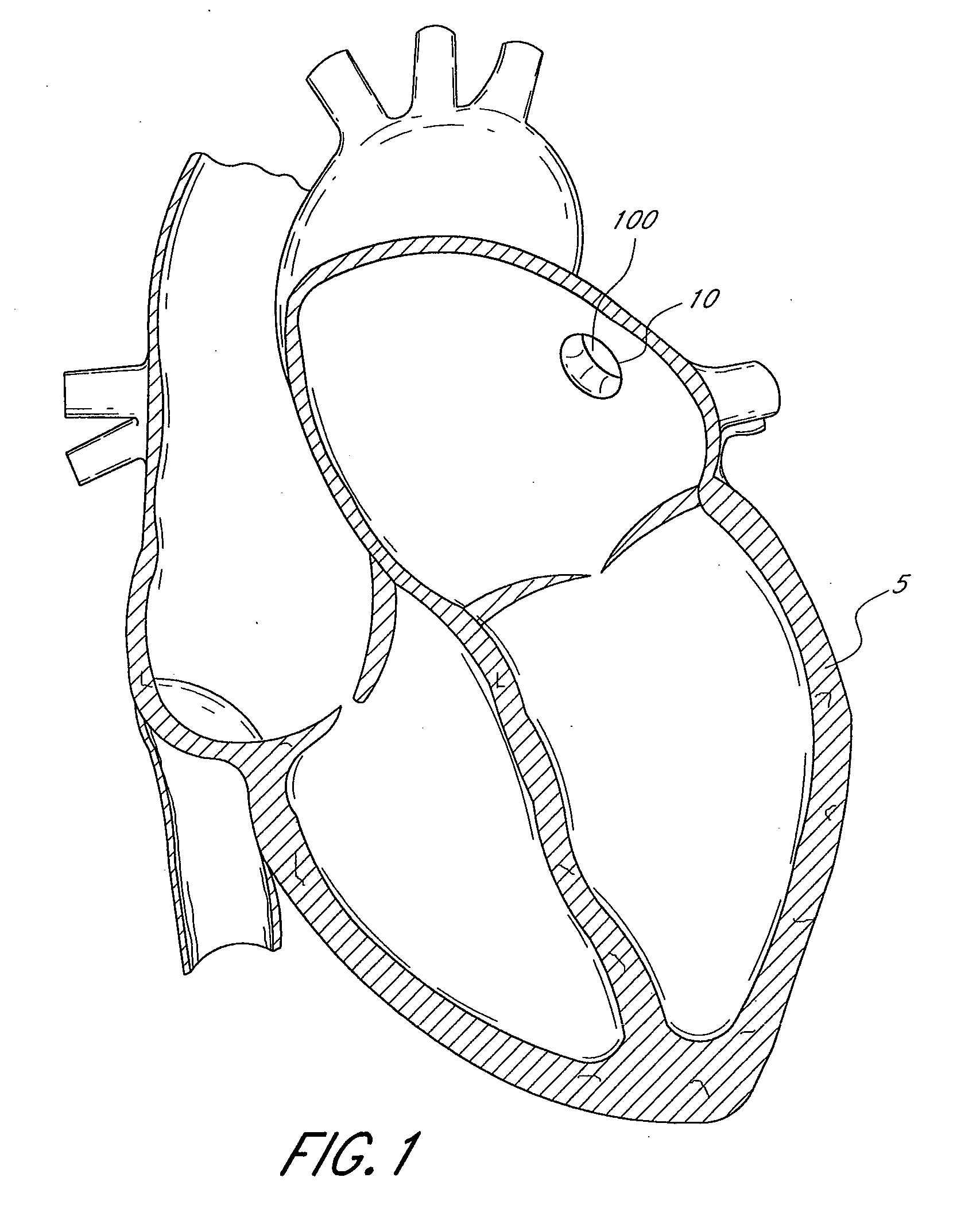
[0063]FIG. 1 illustrates a sectional view of a heart 5 and its left atrial appendage (LAA) 10. An implant 100 is provided at least partially within the LAA 10. The terms “implant”, “occlusion device” or “containment device” are broad terms intended to have their ordinary meaning. In addition, these terms are intended to refer to devices that are inserted into the body. Such devices may include a membrane, barrier and / or cover, or may omit these portions. Embodiments of the invention may also be used to treat other bodily openings, lumen and cavities, besides the LAA 10. For example, in some embodiments, the methods, devices and systems described herein are used to treat any heart opening or defect, such as a patent foramen ovale (PFO), an atrial septal defect (ASD), a ventricular septal defect (VSD), a patent ductus arteriosus (PDA), an aneurysm and / or an aortico-pulmonary window.
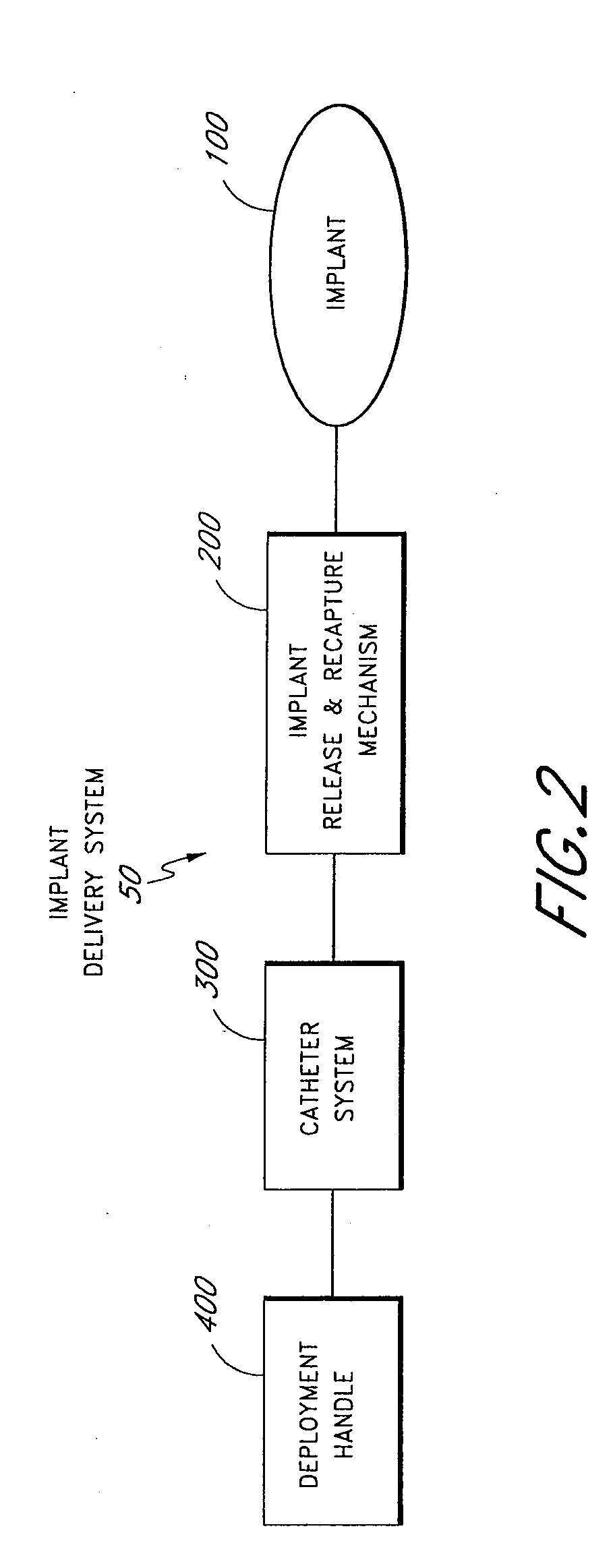
[0064]In various embodiments, an implant 100 can be delivered in a number of ways, e.g., using conventio...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com