Methods and compositions for modulating the activity of the interleukin-35 receptor complex
a technology of interleukin-35 receptor and activity regulation, which is applied in the direction of drug compositions, immunoglobulins, peptides against animals/humans, etc., can solve the problems of undesirable effector t cells, relapse of disease, and numerous harmful side effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
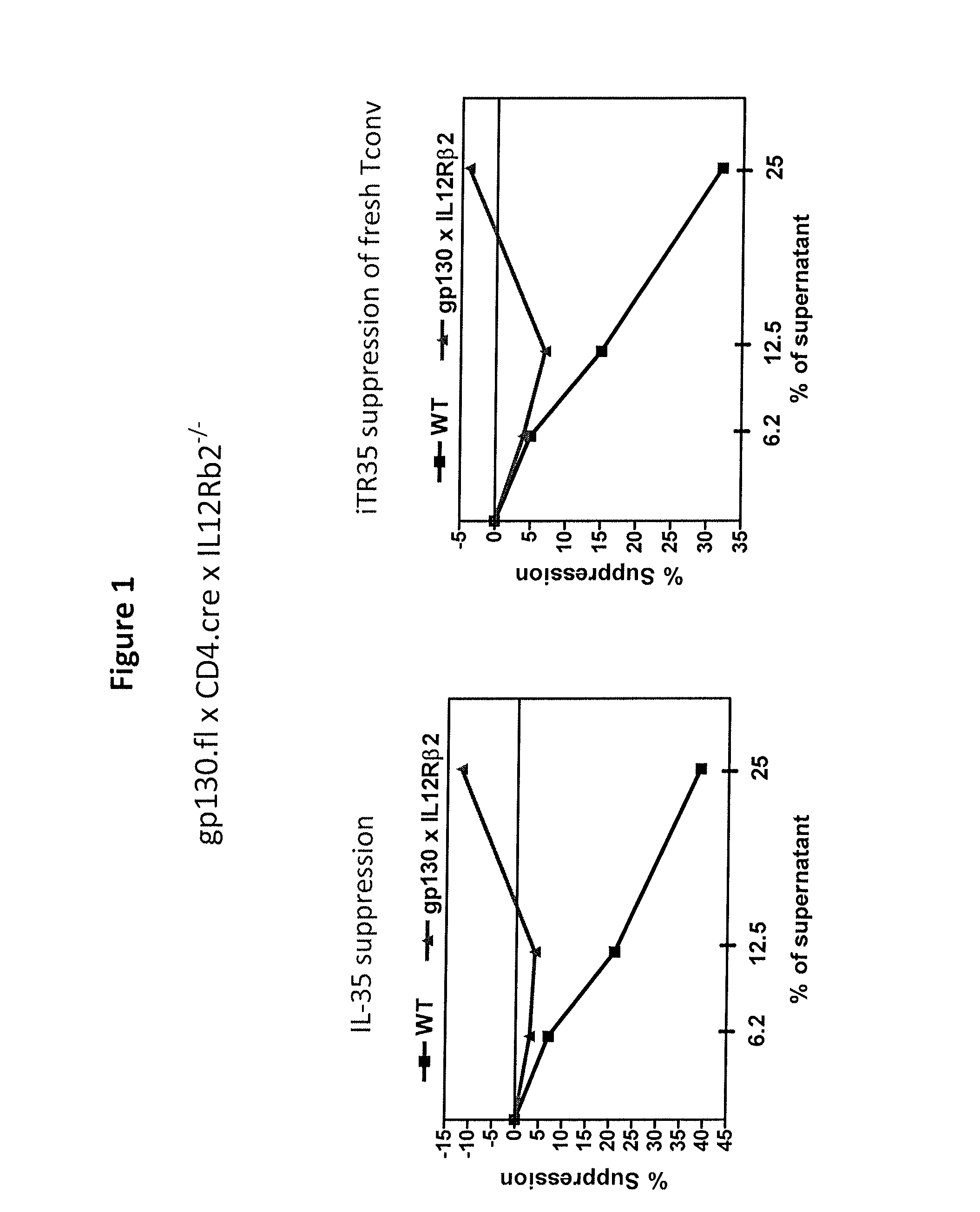
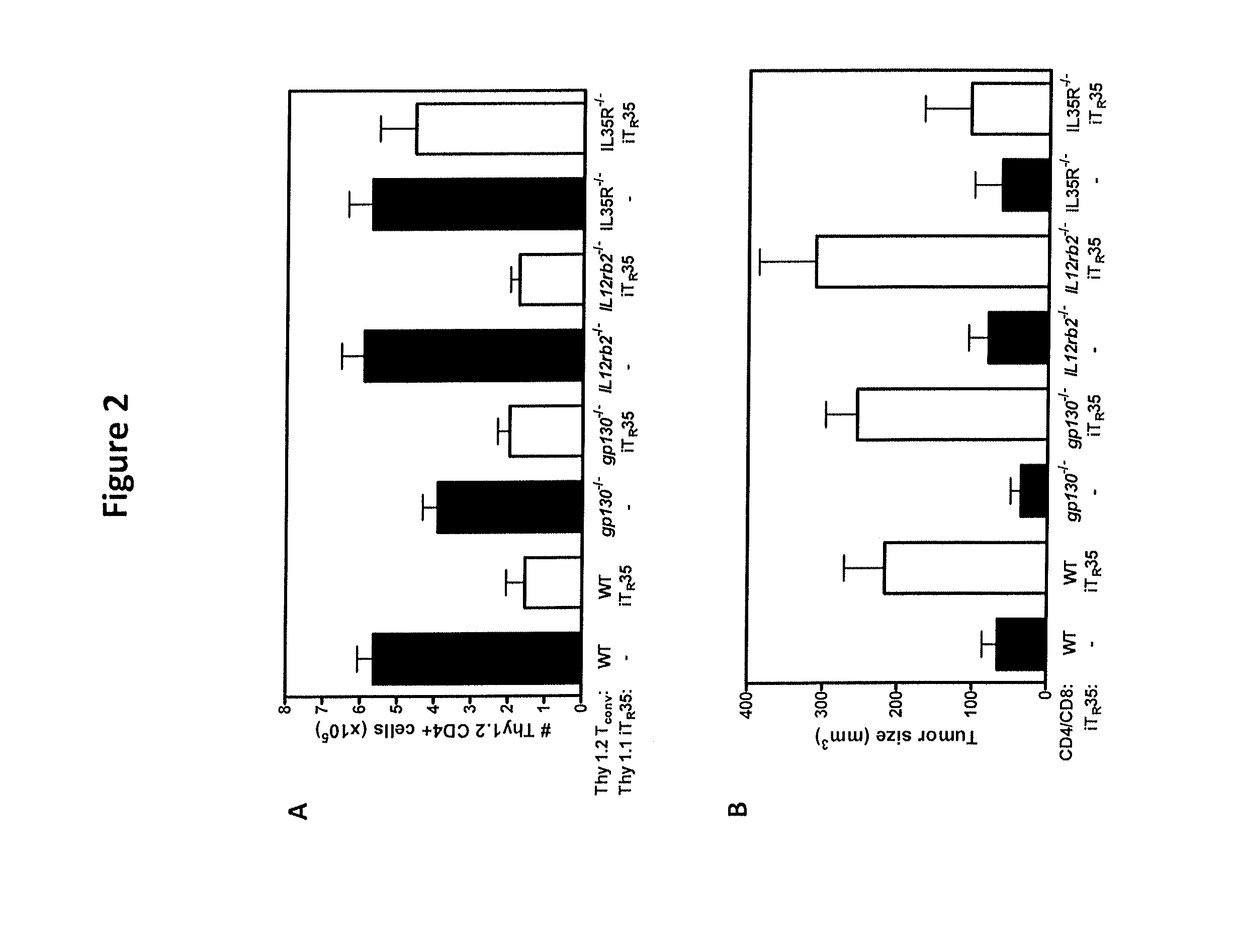
IL-35 Signaling and Suppression Mediated by IL-35 Require the Expression of the IL-35R
Materials and Methods:
[0176]Mice. C57BL / 6 (wild type), CD4.cre, and IL12Rβ2− / − mice were purchased from the Jackson Laboratory. Gp130 floxed knockin mice were provided by Rodger McEver at Oklahoma Medical Research Foundation. gp130 fl×CD4.cre×IL12Rb2− / − were obtained by breeding the three mouse strains listed. All animal experiments were performed in American Association for the Accreditation of Laboratory Animal Care-accredited, specific-pathogen-free facilities in the St. Jude Animal Resource Center following national, state and institutional guidelines. Animal protocols were approved by the St Jude Animal Care and Use Committee.
[0177]Tconv Cell Purification. Teff(CD4+CD25−CD45RBhi) from the spleens and lymph nodes of C57BL / 6 or knockout age-matched gp130.fl×CD4.cre×IL12Rb2− / − mice were positively sorted by FACS. After red blood cell lysis, cells were stained with antibodies against CD4, CD25 and...
example 2
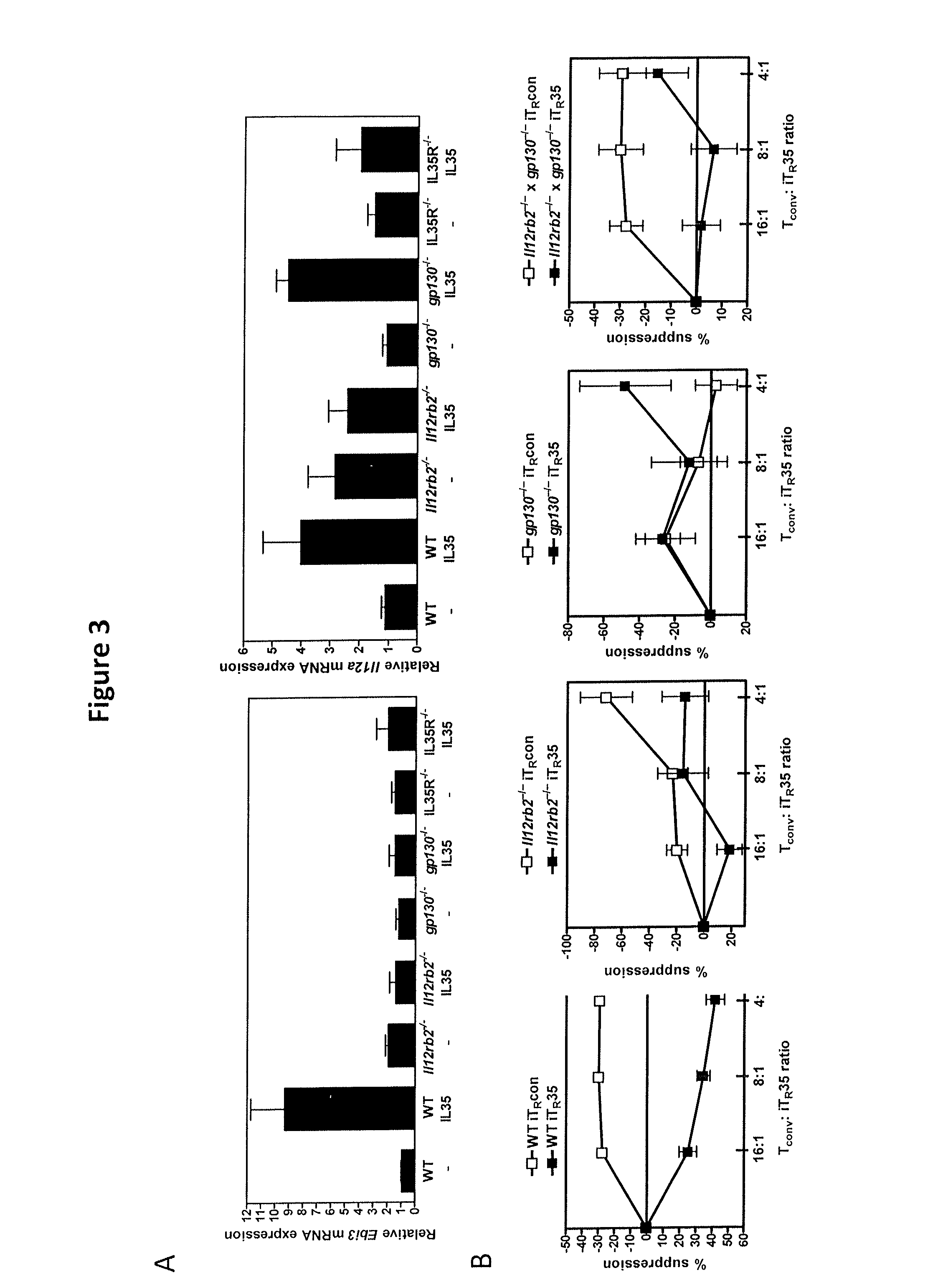
IL-35 Signals Primarily Through Two Different STAT Proteins, STAT1 and STAT4
Materials and Methods:
[0182]Mice. Spleens and lymph nodes from Il12rb1− / − mice were provided by D. Fairweather and J. A. Frisancho (Johns Hopkins University), CD4cre×gp130fl / fl mice were provided by M. Karin and S. Grivennikov (University of California at San Diego), IL27ra− / − mice were provided by C. Hunter and J. Stumhofer (University of Pennsylvania), Stat1− / − mice were provided by A. Satoskar and P. Reville (Ohio State University), and Stat3− / − mice were provided by C. Drake and H.R. Yen (Johns Hopkins University). IL12rb2− / −, Stat4− / −, Rag1− / −, C57BL / 6, B6.PL and Balb / c mice were purchased from the Jackson Laboratory. All animal experiments were performed in American Association for the Accreditation of Laboratory Animal Care-accredited, specific-pathogen-free facilities in the St. Jude Animal Resource Center following national, state and institutional guidelines. Animal protocols were approved by the S...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Fraction | aaaaa | aaaaa |
| Composition | aaaaa | aaaaa |
| Length | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com