Aluminum electroplating solution
a technology of aluminum electroplating and solution, applied in the field of aluminum electroplating solution, can solve the problems of difficult conductivity of aluminum electroplating in aqueous solution type plating bath, shortening the bath life, and reducing current efficiency, so as to achieve enhanced precipitation efficiency of plating and improve the uniformity of film thickness
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
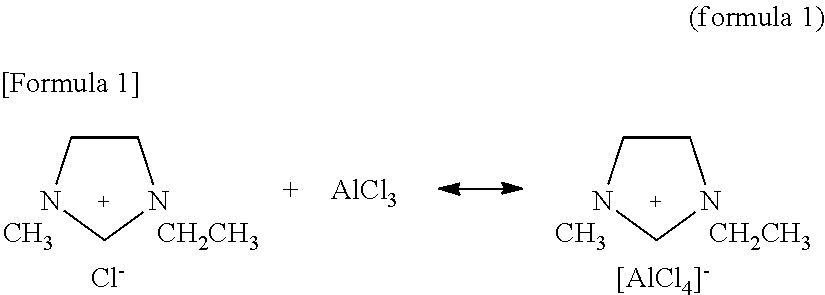
[0028]1-ethyl-3-methylimidazolium chloride (commercially available from KANTO CHEMICAL CO., INC.; [EMIM]Cl) and anhydrous aluminum chloride (Wako Pure Chemical Industries, Ltd., AlCl3) were used. A weighing capacity was conducted in a glove box at a humidity set to 5% and a temperature set to 25° C., and AlCl3 was added so as to be a molar ratio of [EMIM]Cl:AlCl3=1:3 to obtain a 100 ml melt. The above mentioned melt was dissolved in 300 ml toluene (Wako Pure Chemical Industries, Ltd.), and a plating solution was prepared so as to be 400 ml in a total volume. The resulting electrolyte 400 ml was charged into a polypropylene-made electrolytic bath having longitudinal×transversal×height of 100 mm×50 mm×100 mm.
[0029]Next, an aluminum plate of purity 99.9% having longitudinal×transversal of 75 mm×75 mm and a thickness of 2 mm, as an anode electrode, and a copper foil having longitudinal×transversal of 50 mm×50 mm and a thickness of 0.1 mm, as a cathode electrode, were facing-positioned a...
examples 2 to 12
[0034]Aluminum chloride salts AlCl3 were dissolved by using organic compounds and organic solvents shown at Table 1 so as to become one of several salt concentrations A (mol / l), to prepare plating solutions of Examples 2 to 12 as well as in Example 1.
[0035]An aluminum electroplating was conducted at an electric current density −10 mA / cm2 for 20 minutes or an electric current density −20 mA / cm2 for 10 minutes by using a constant electric current source. Then, an evaluation of a current efficiency and an observation of a surface state of the plating film were conducted. It was conducted at a voltage of 3V or less in the plating. The current efficiency was determined by determining a precipitation amount of aluminum by actual measurement, comparing this to a precipitation amount precalculated based on an electric current value of a coulomb meter, and determining a ratio to the latter precipitation amount calculated as a percentage. The results are shown at Table 1.
[0036]As clear from t...
example 13
[0037]According to the plating method as well as in Example 1, a plating was conducted onto a material to be plated which is a copper foil having a center folded at 90 degree in the L-shape. In result of measuring film thicknesses of five portions of a copper foil face, good results of a plating film thickness of 4 μm and a distribution within 8% were obtained. The current efficiency was as good as 97%.
[0038]As clear from these results, it was found that, when the solvent and the solute as in the present Example were used, a large electric current of a electroplating can be conducted, a plating of aluminum can be achieved efficiently and in a short period of time, and parts having a uniform plating applied were able to be provided even from a steric shape.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Fraction | aaaaa | aaaaa |
| Molar ratio | aaaaa | aaaaa |
| Dielectric constant | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com