Combination comprising an atp analog and an adenosine receptor antagonist or a nucleobase/nucleoside analog for the treatment of cancer
a cancer and atp technology, applied in the field of cancer treatment, can solve the problems of unsatisfactory explanation of tki-induced diarrhea, difficult to achieve side effects, and failure of most of these studies
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
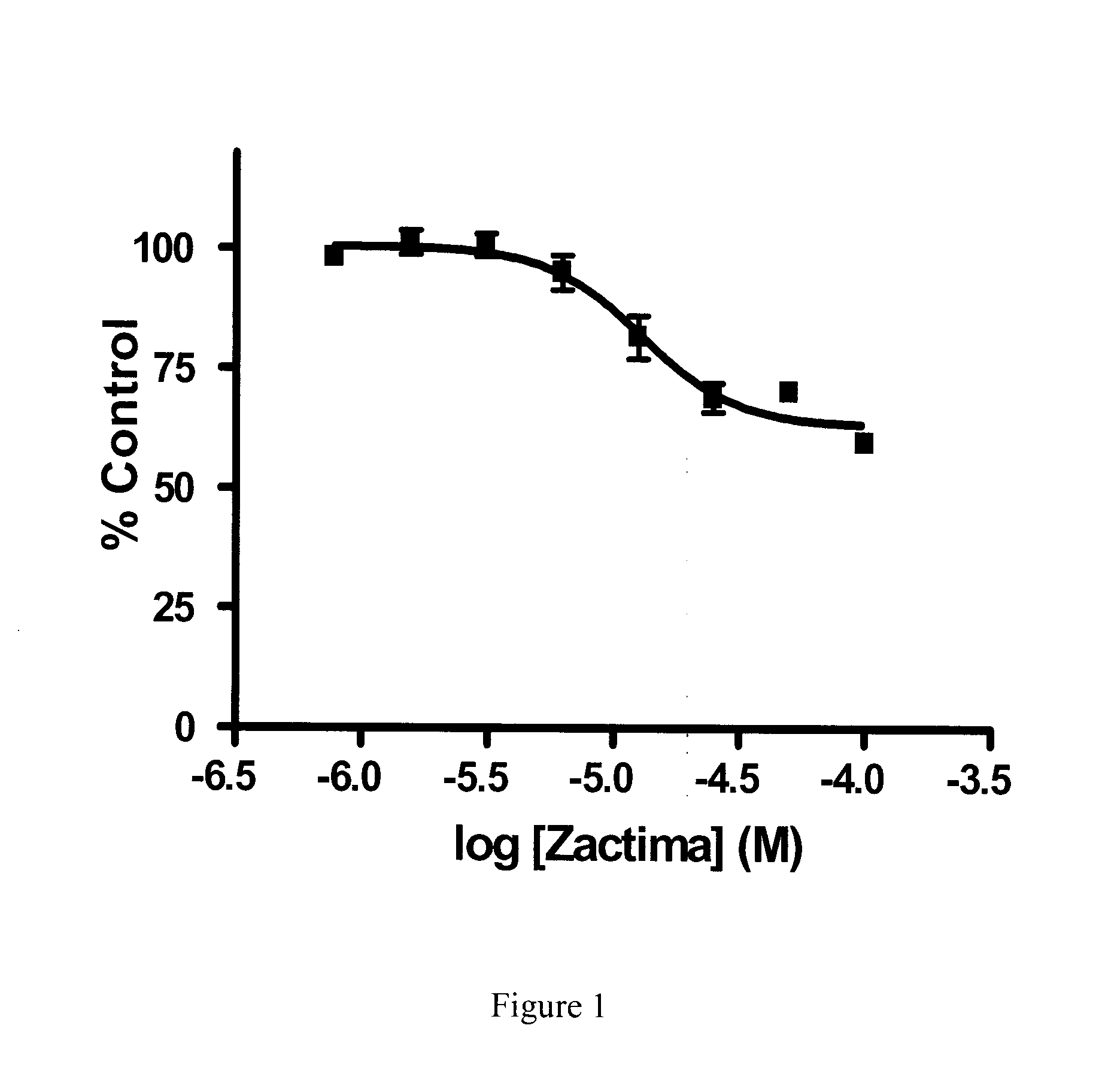
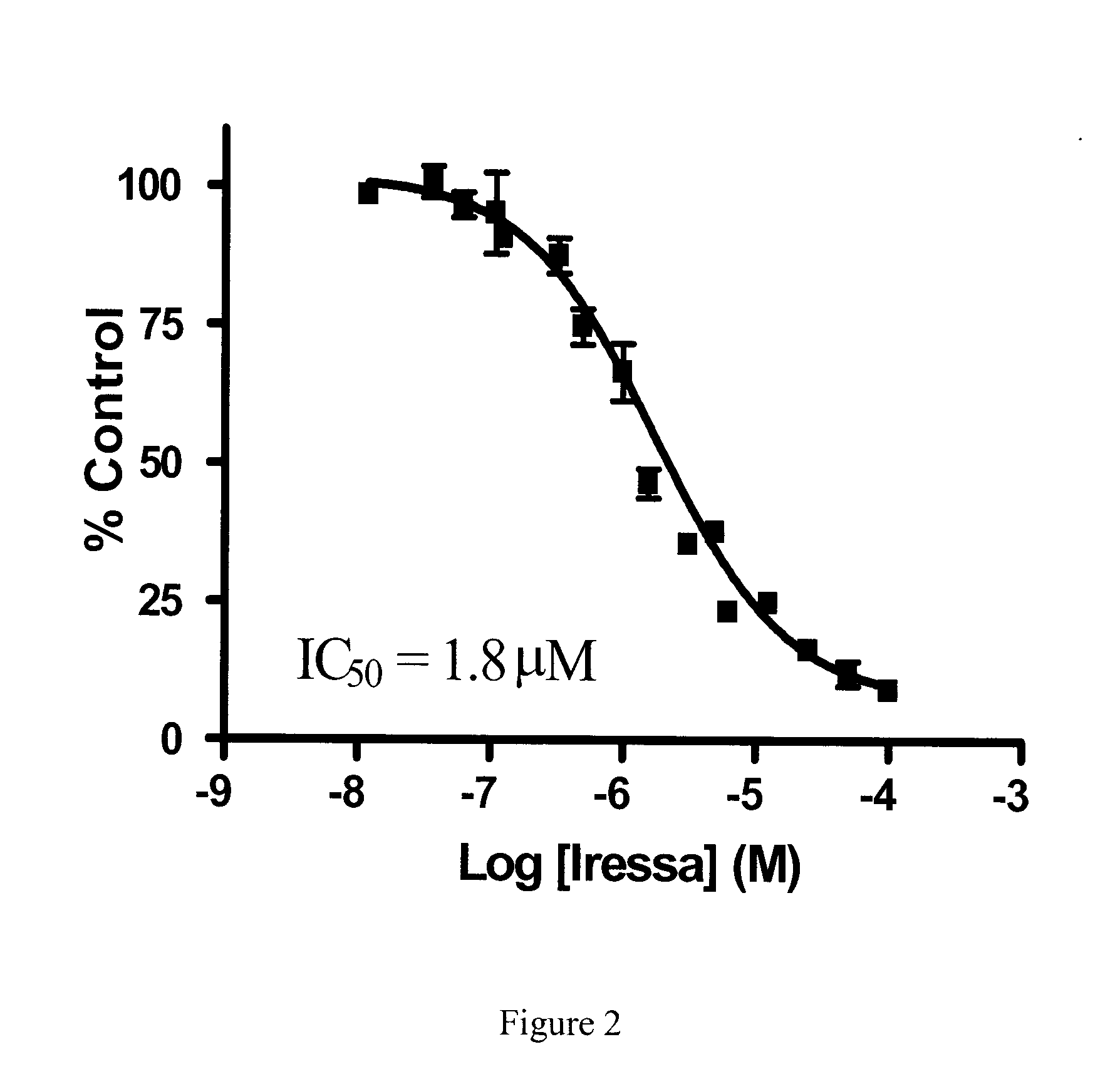
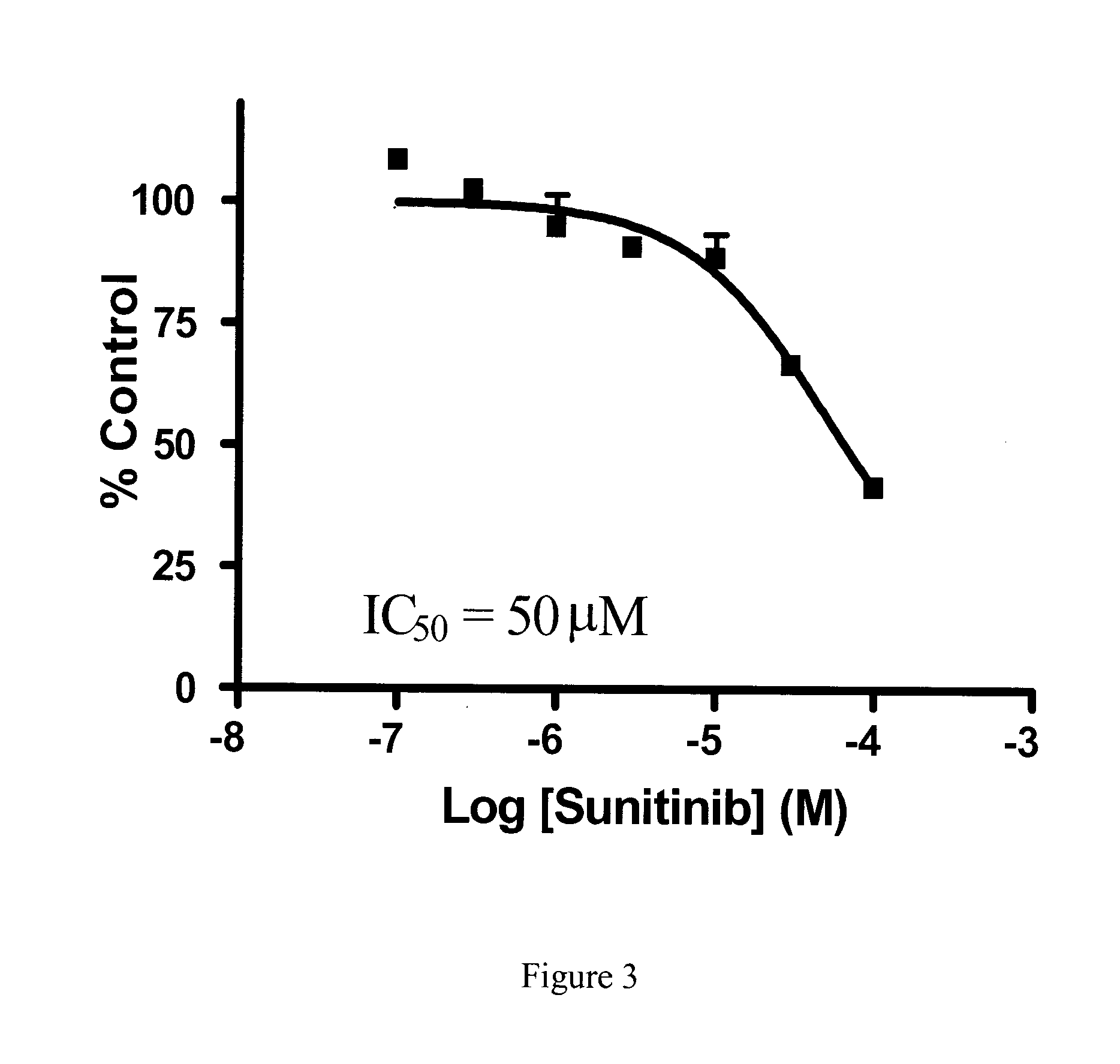
Effect of Zactima (Vandetanib), Iressa (Gefitinib) and Sutent (Sunitinib) on Nucleobase Transport
[0152]Human renal proximal tubule cells (hRPTCs) were seeded in 12-well plates at densities of 105 cells / well and grown for 7 days with medium changes every 3-4 days. On the day of experiments, growth medium was aspirated and cells were washed twice with 1.5 ml of 20 mM Tris-HCl buffer (pH 7.4) containing 150 mM NaCl, 1.2 mM MgCl2 and 1 mM CaCl2 (hereafter referred to as sodium buffer).
[0153]Inhibition of [3H] adenine transport in hRPTCs by the TKIs vandetanib, gefitinib and sunitinib was investigated by incubating hRPTC cultures with 1 μM [3H]adenine in sodium buffer at pH 7.4 in the absence or presence of graded concentrations of vandetanib, gefitinib and sunitinib. At the end of 2 minute incubations, cells were washed twice with sodium buffer and intracellular accumulation of [3H]adenine was determined by solubilizing cells with 5% Triton-X-100 and subsequent determination of radioact...
example 2
FOLFOX Regimen Including a TKI
[0155]An exemplary FOLFOX regimen including a TKI such as cediranib is described below:
Day 1:
[0156]Leucovorin 200 mg / m2 (0-2 hrs)
[0157]Oxaliplatin 85 mg / m2 (0-2 hrs)
[0158]5-Fluorouracil (5-FU) 400 mg / m2 (bolus at 2 hrs)
[0159]5-FU 600 mg / m2 (2-22 hrs)
Day 2:
[0160]Leucovorin 200 mg / m2 (0-2 hrs)
[0161]5-FU 400 mg / m2 (bolus at 2 hrs)
[0162]5-FU 600 mg / m2 (2-22 hrs)
Day 3-Day 10:
[0163]TKI such as Cediranib (30 mg / day)
Day 11-14:
[0164]No treatment
Day 15:
[0165]Cycle 2 starts
example 3
Effects of Adenosine, Adenosine Receptor Antagonists, and TKIs on Human Nucleoside Equilibrative Transporters (hENTS)
[0166]Introduction:
[0167]T84 cells are used as a model for epithelial chloride (Cl−) secretion (Strohmeier et al., J Biol Chem 1995, 270, 2387). Matthews et al. (Surgery 1994, 116, 150; J Clin Invest 1995, 96, 117) showed that adenosine released from cultured intestinal epithelial cells during hypoxia activates the Cl− secretion via activation of the adenosine receptors (A2b) (Stehle et al., Mol Endocrinol 1992, 6, 384). Tally et. al, (Surgery 1996, 120, 248) showed that human equilibrative nucleoside transporters (hENTs) localized on intestinal epithelial cells scavenge free adenosine thus regulating the concentrations of adenosine available to interact with adenosine receptors (A2b) localized on intestinal cells leading to activation of Cl− secretion and adenosine induced diarrheal response.
[0168]Methods:
[0169]T84 cells were routinely grown in DMEM / F12 (1:1) cell cu...
PUM
| Property | Measurement | Unit |
|---|---|---|
| pH | aaaaa | aaaaa |
| adenosine induced currents | aaaaa | aaaaa |
| structure | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com