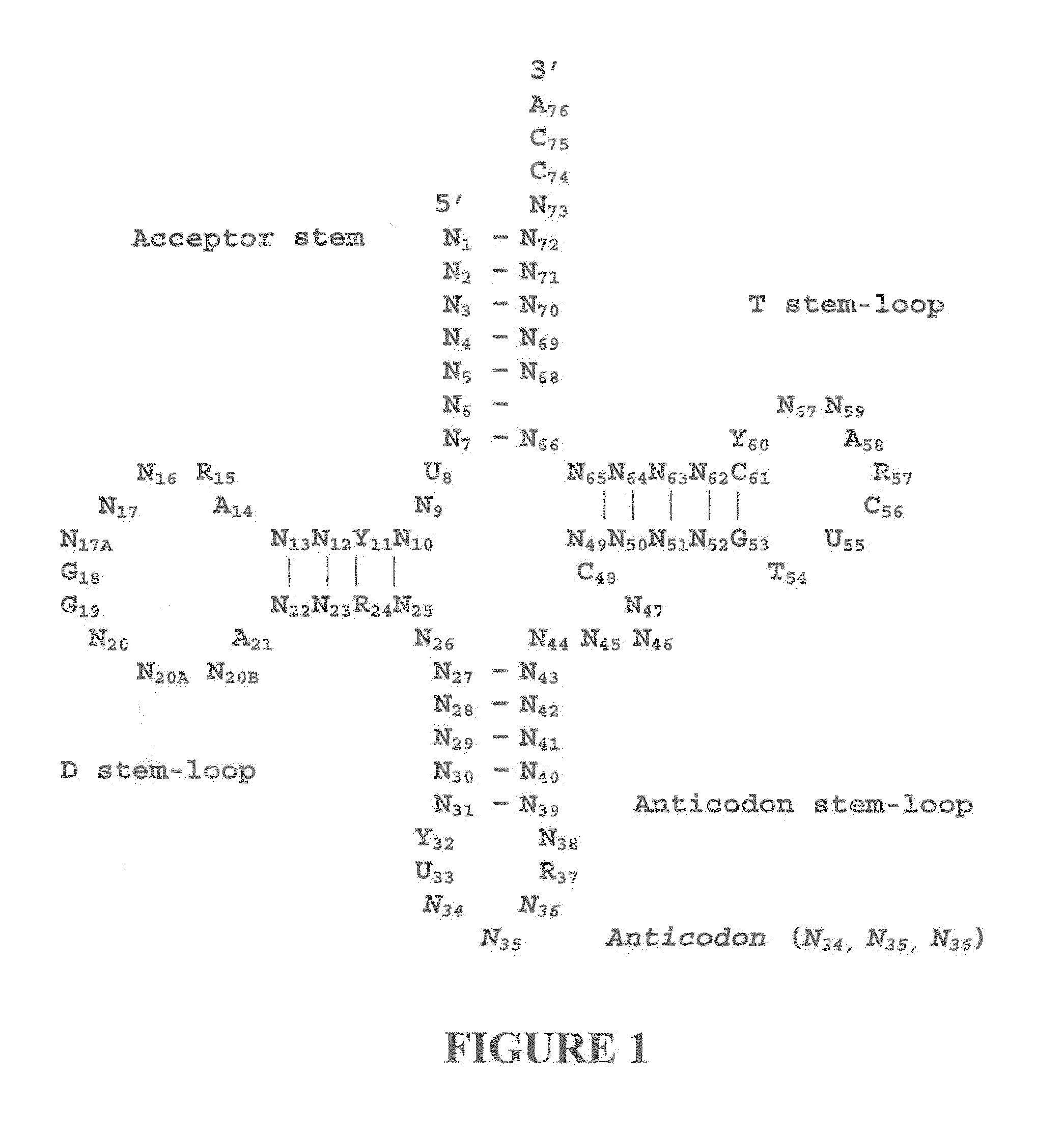Protein synthesis modulators
a technology of ribosomes and modulators, applied in the field of ribosome modulators, can solve the problems of cancerous cells, particularly problematic resistance to anti-infective agents, and the evolution of strains of cells or organisms resistant to currently effective therapeutic agents, and achieve the effect of modulating ribosomal activity and ribosomal activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Three-Dimensional Structure of a RNA Microhelix when Associated with the Large Ribosomal Subunit
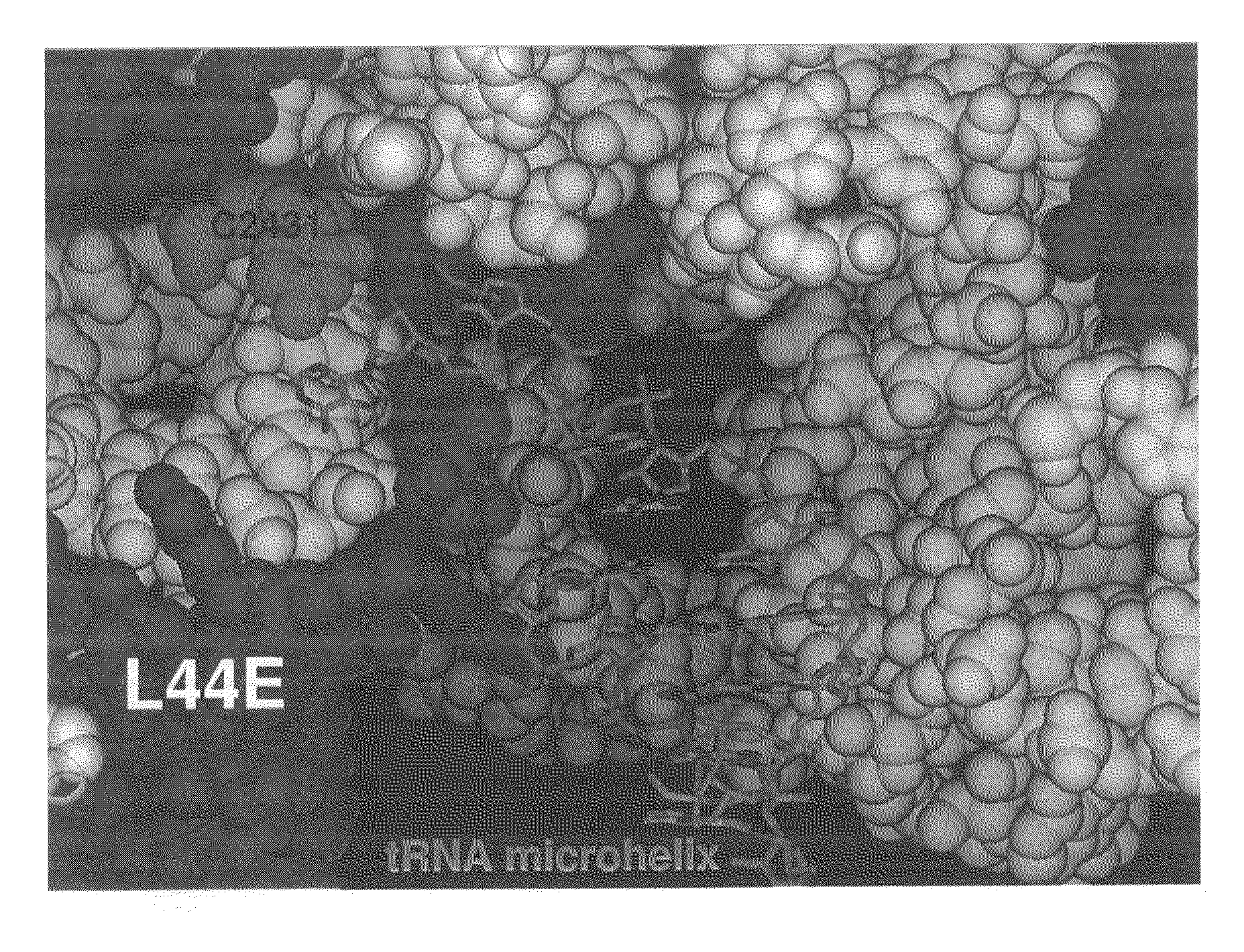
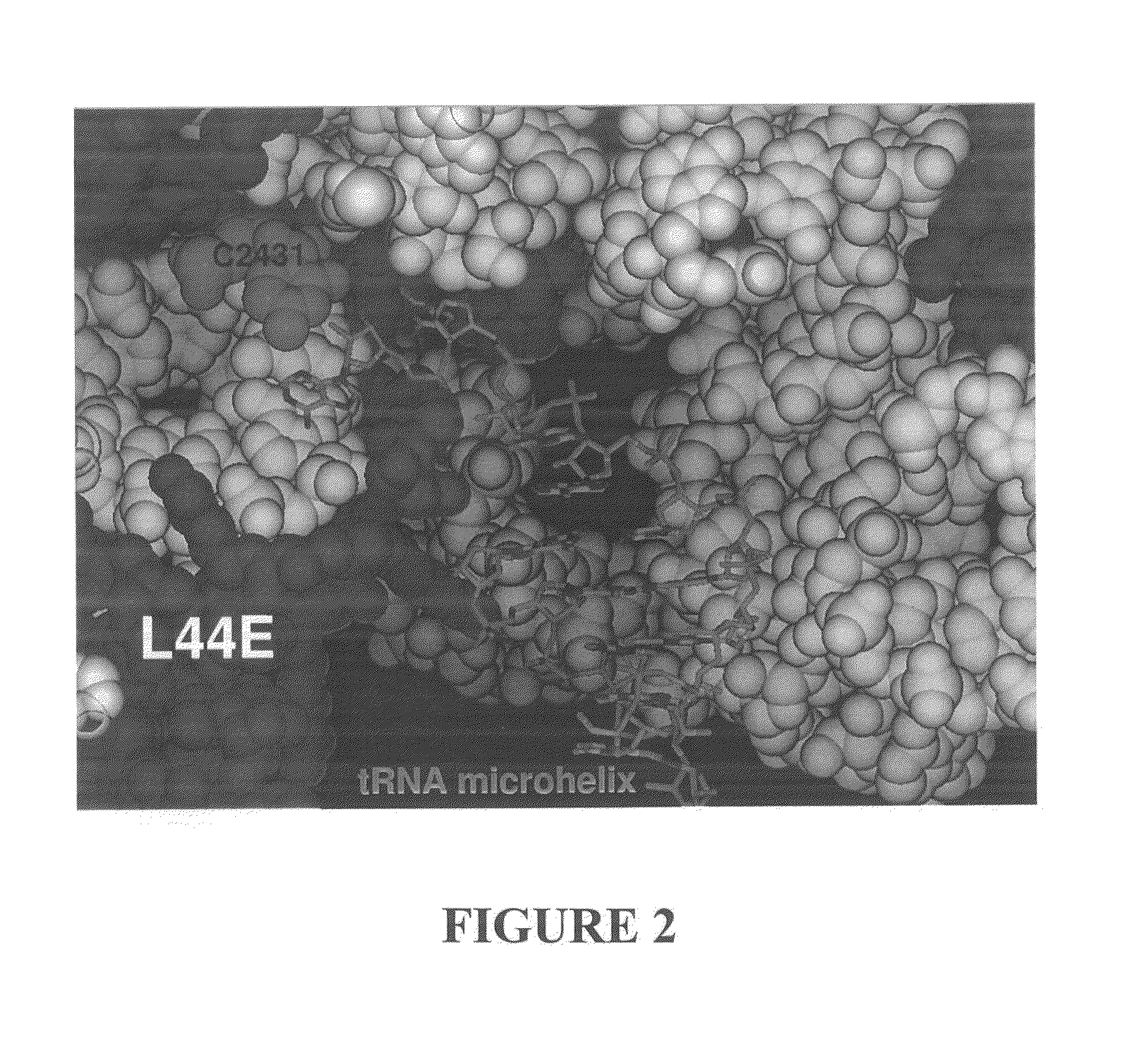
[0181]Ribosomes were purified and crystallized as described previously (U.S. Patent Application Publication No. US 2002 / 0086308 A1). Crystals containing H. marismortui large ribosomal subunits complexed with the RNA microhelix of Formula I, were obtained by soaking pre-formed, large-subunit crystals in stabilizing buffers containing the RNA microhelix of Formula I.
[0182]The RNA microhelix was solubilized in standard stabilization buffer (Ban et al. (2000) Science 289: 905-920) to a final concentration of about 4.0 mM, and then incubated at 4° C. for 18 hours prior to cryo-vitrification of crystals in liquid propane. X-ray diffraction data were collected at Beamline 14-4 at the European Radiation Synchrotron Facility in Grenoble, France. Data were reduced using denzo or HKL2000 software and scaled with scalepack (Otwinowski (1997) “Processing of X-ray Diffraction Data Collected In Oscillat...
example 2
Three-Dimensional Structure of a RNA Microhelix when Associated with the Large Ribosomal Subunit
[0184]Ribosomes were purified and crystallized as described previously (U.S. Patent Application Publication No. US 2002 / 0086308 A1). Crystals containing H. marismortui large ribosomal subunits complexed with the RNA microhelix of Formula II, were obtained by soaking pre-formed, large-subunit crystals in stabilizing buffers containing the RNA microhelix of Formula II.
[0185]The RNA microhelix was solubilized in standard stabilization buffer (Ban et al. (2000) supra) to a final concentration of about 5.0 mM, and then incubated at 4° C. for 18 hours prior to cryo-vitrification of crystals in liquid propane. X-ray diffraction data were collected at Beamline X25 at Brookhaven National Laboratory in Upton, N.Y. Data were reduced using denzo or HKL2000 software and scaled with scalepack (Otwinowski (1997) “Processing of X-ray Diffraction Data Collected In Oscillation Mode,”Methods in Enzymology 2...
example 3
Additional Three-Dimensional Structures of RNA Microhelices when Associated with the Large Ribosomal Subunit
[0187]Using the general procedures of Example 1, crystals containing H. marismortui large ribosomal subunits complexed with other RNA microhelices (for example, a 5′ ACCA 3′ RNA microhelix) are obtained. The three-dimensional structures of these crystal complexes can be obtained as described in Examples 1 and 2. The resulting structures can also be used to design other candidate molecules useful as protein synthesis modulators.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com