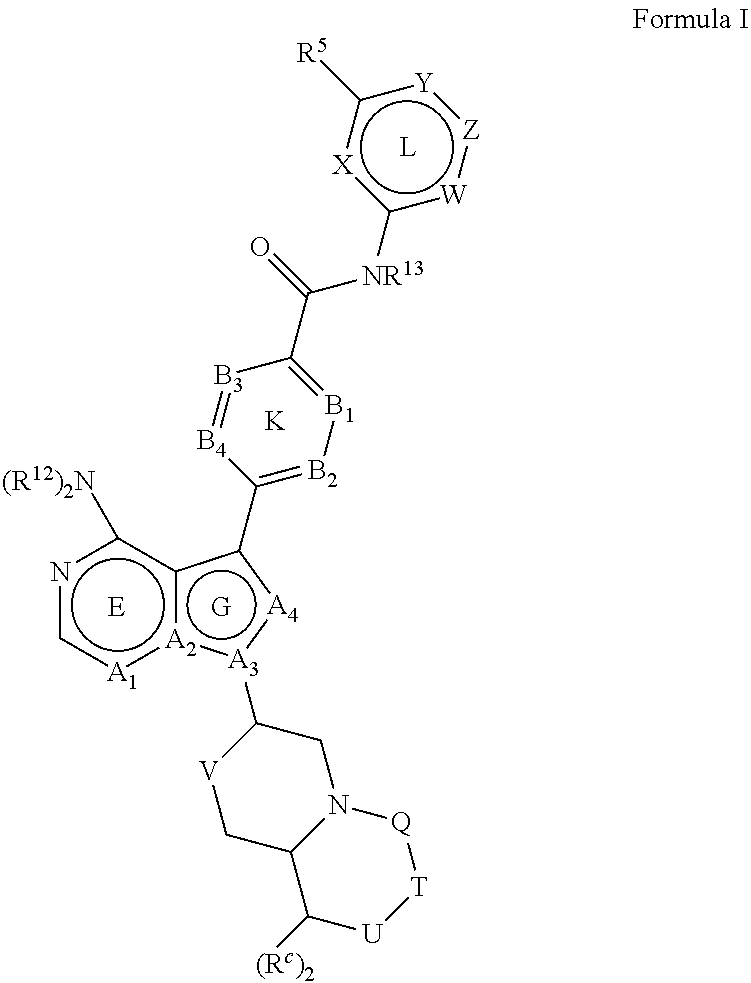
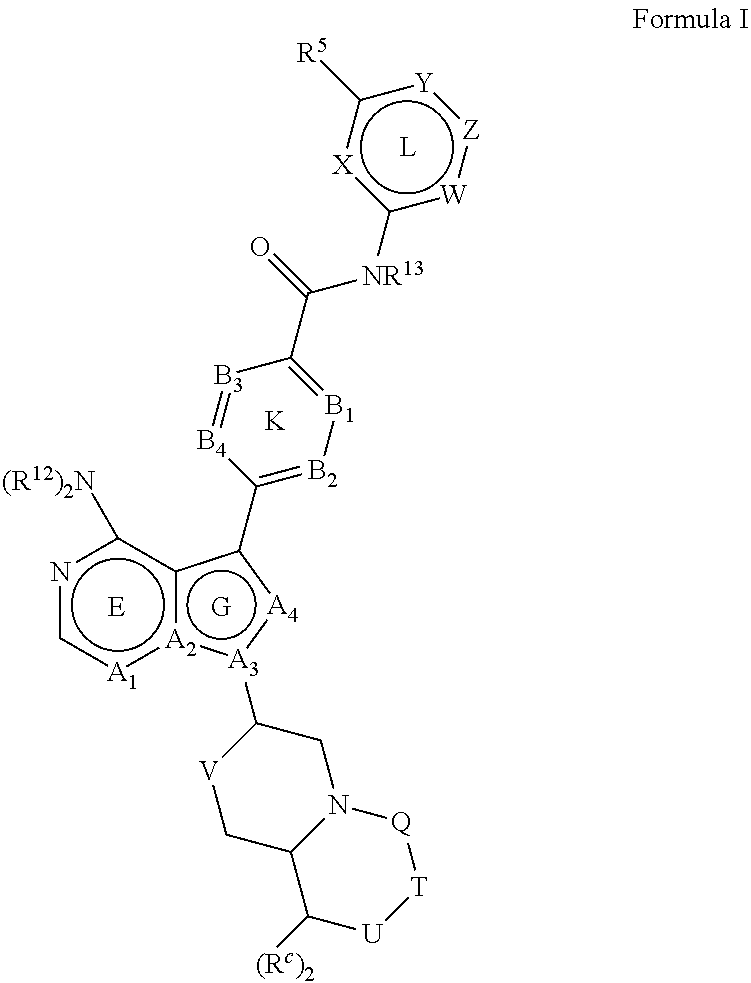
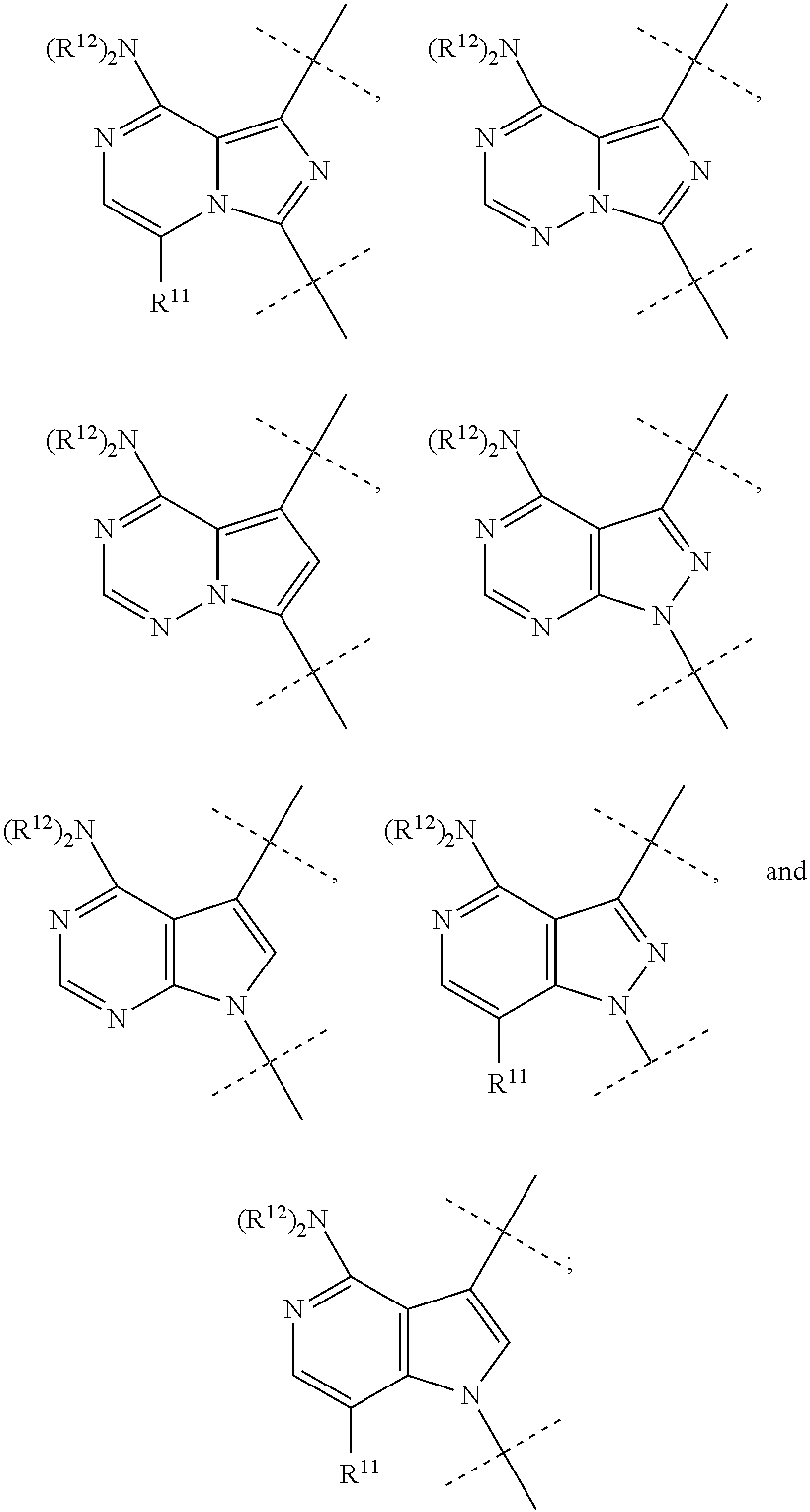
Btk inhibitors
a technology of btk inhibitors and inhibitors, which is applied in the direction of biocide, drug composition, immunological disorders, etc., can solve the problems of serious adverse effects, fyn-deficient mice also show pronounced neurological defects, and are prohibitive for the development of btk inhibitors
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
[1018]
4-(8-amino-3-((6-S,8aS)-3-oxohexahydro-1H-oxazolo[3,4-a]pyridin-6-yl)imidazo[1,5-a]pyrazin-1-yl)-N-(4-(trifluoromethyl)pyridin-2-yl)benzamide
(a) 4-(8-((2,4-dimethoxybenzyl)amino)-3-((3S,6S)-6-(hydroxymethyl)piperidin-3-yl)imidazo[1,5-a]pyrazin-1-yl)-N-(4-(trifluoromethyl)pyridin-2-yl)benzamide
[1019]To a solution of ((2S,5S)-5-(8-((2,4-dimethoxybenzyl)amino)-1-(4-((4-(trifluoromethyl) pyridin-2-yl)carbamoyl)phenyl)imidazo[1,5-a]pyrazin-3-yl)piperidin-2-yl)methyl acetate (120 mg, 0.17 mmol) in 3 mL of MeOH was added NaOMe (46 mg, to 0.85 mmol). The reaction mixture was stirred at room temperature for 3 h under N2.
[1020]The mixture was poured into aq. NH4Cl, extracted with DCM. The organic layer was dried over Na2SO4, and concentrated in vacuo to give 110 mg of crude 4-(8-((2,4-dimethoxybenzyl)amino)-3-((3S,6S)-6-(hydroxymethyl)piperidin-3-yl)imidazo[1,5-a]pyrazin-1-yl)-N-(4-(trifluoromethyl)pyridin-2-yl)benzamide, which was used in the next step directly. MS-ESI (m / z): 662.0 (M+...
example 2
[1023]
4-(8-amino-3-((6R,8aR)-3-oxohexahydro-1H-oxazolo[3,4-a]pyridin-6-yl)imidazo[1,5-a]pyrazin-1-yl)-N-(4-(trifluoromethyl)pyridin-2-yl)benzamide
(a) 4-(8-((2,4-dimethoxybenzyl)amino)-3-((6R,8aR)-3-oxohexahydro-1H-oxazolo[3,4-a]pyridin-6-yl)imidazo[1,5-a]pyrazin-1-yl)-N-(4-(trifluoromethyl)pyridin-2-yl)benzamide
[1024]To a mixture of (2R,5R)-benzyl 5-(8-((2,4-dimethoxybenzyl)amino)-1-(44(4-(trifluoromethyl)pyridin-2-yl)carbamoyl)phenyl)imidazo[1,5-a]pyrazin-3-yl)-2-(hydroxymethyl)piperidine-1-carboxylate (40 mg) in 3 mL of MeOH was added NaOMe (54 mg, 1 mmol). The reaction mixture was stirred at room temperature for 24 h, and then at 40° C. for 6 hours under N2. The mixture was poured into aq. NH4Cl, extracted with DCM (20 mL). The organic layer was dried over Na2SO4, and concentrated in vacuo to give 40 mg crude of 4-(8-((2,4-dimethoxybenzyl)amino)-3-((6R,8aR)-3-oxohexahydro-1H-oxazolo[3,4-a]pyridin-6-yl)imidazo[1,5-a]pyrazin-1-yl)-N-(4-(trifluoromethyl)pyridin-2-yl)benzamide, which...
example 3
[1026]
4-(8-amino-3-((6R,8aS)-2-methyl-3-oxooctahydroimidazo[1,5-a]pyridin-6-yl)imidazo[1,5-a]pyrazin-1-yl)-N-(4-(trifluoromethyl)pyridin-2-yl)benzamide
(a) (2S,5R)-benzyl5-(8-amino-1-(4-((4-(trifluoromethyl)pyridin-2-yl)carbamoyl)phenyl)imidazo[1,5-a]pyrazin-3-yl)-2-(methoxymethyl)piperidine-1-carboxylate
[1027]To a degassed mixture of (2S,5R)-benzyl 5-(8-amino-1-bromoimidazo[1,5-a]pyrazin-3-yl)-2-(methoxymethyl)piperidine-1-carboxylate (50 mg, 0.105 mmol), 4-(4,4,5,5-Tetramethyl-[1,3,2]dioxaborolan-2-yl)-N-(4-trifluoromethyl-pyridin-2-yl)-benzamide (41 mg, 0.105 mmol) and K2CO3 (44 mg, 0.316 mmol) in dioxane / H2O (6 mL, 3:1) was added Pd(dppf)Cl2 under N2. The mixture was heated to 100° C. for 1 hour. The reaction mixture was cooled to room temperature and filtered. The filtrate was concentrated, and the residue was purified on silica gel column chromatograph (PE:EA=100%˜70%) to give (2S,5R)-benzyl 5-(8-amino-1-(4-((4-(trifluoromethyl)pyridin-2-yl)carbamoyl)phenyl)imidazo[1,5-a]pyrazi...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Electric charge | aaaaa | aaaaa |
| Electric charge | aaaaa | aaaaa |
| Electric charge | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com