Single lentiviral vector system for induced pluripotent (IPS) stem cells derivation
a single lentiviral vector and stem cell technology, applied in the direction of viruses/bacteriophages, peptides, genetically modified cells, etc., can solve the problems of limiting the potential clinical transplantation potential of patients, limiting the future use of patient-specific autologous escs, etc., to improve the development potential of ips cells, and improve the effect of ips cell derivation efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
A Single Lentiviral Vector for the Expression of a Stem Cell Cassette
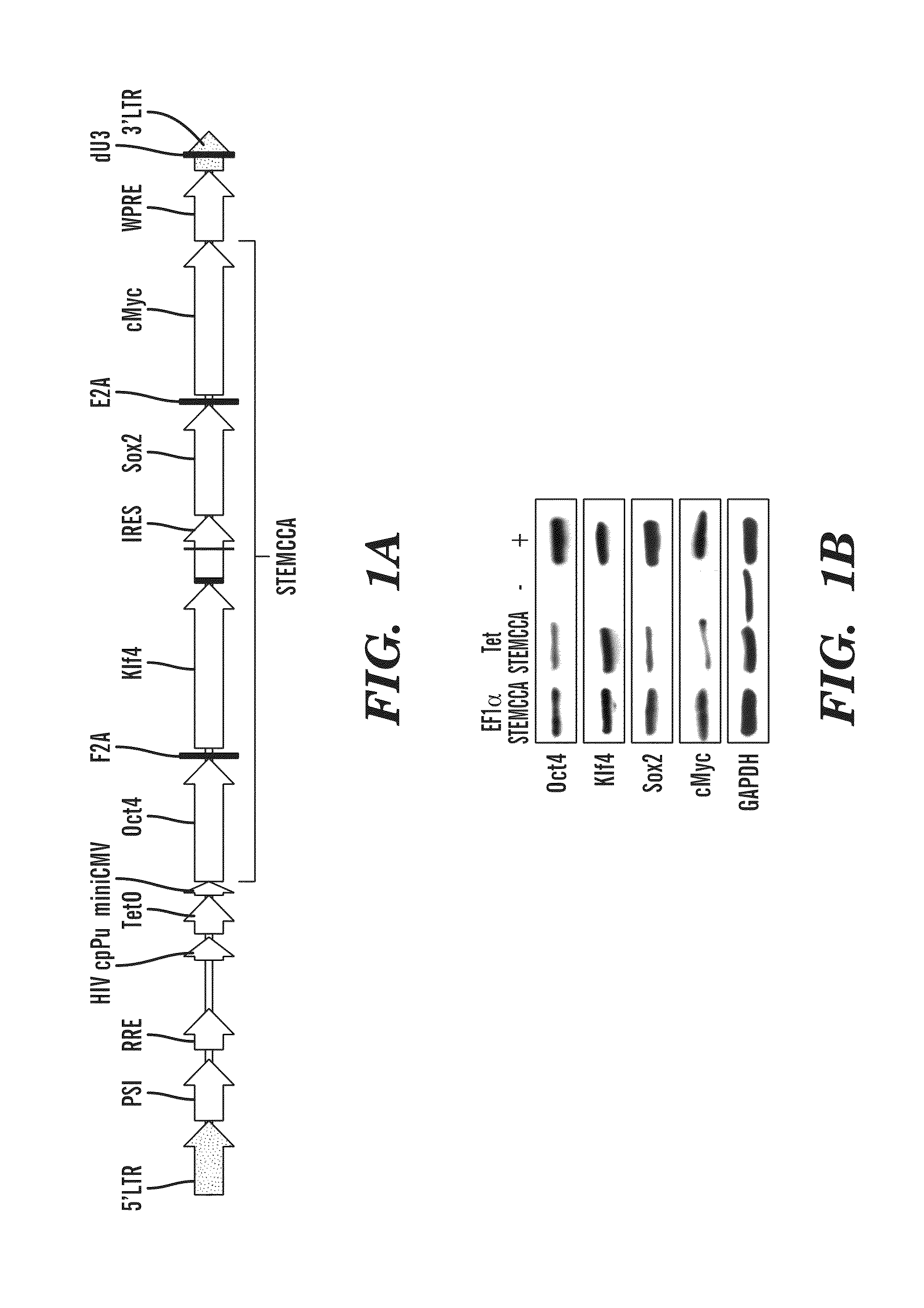
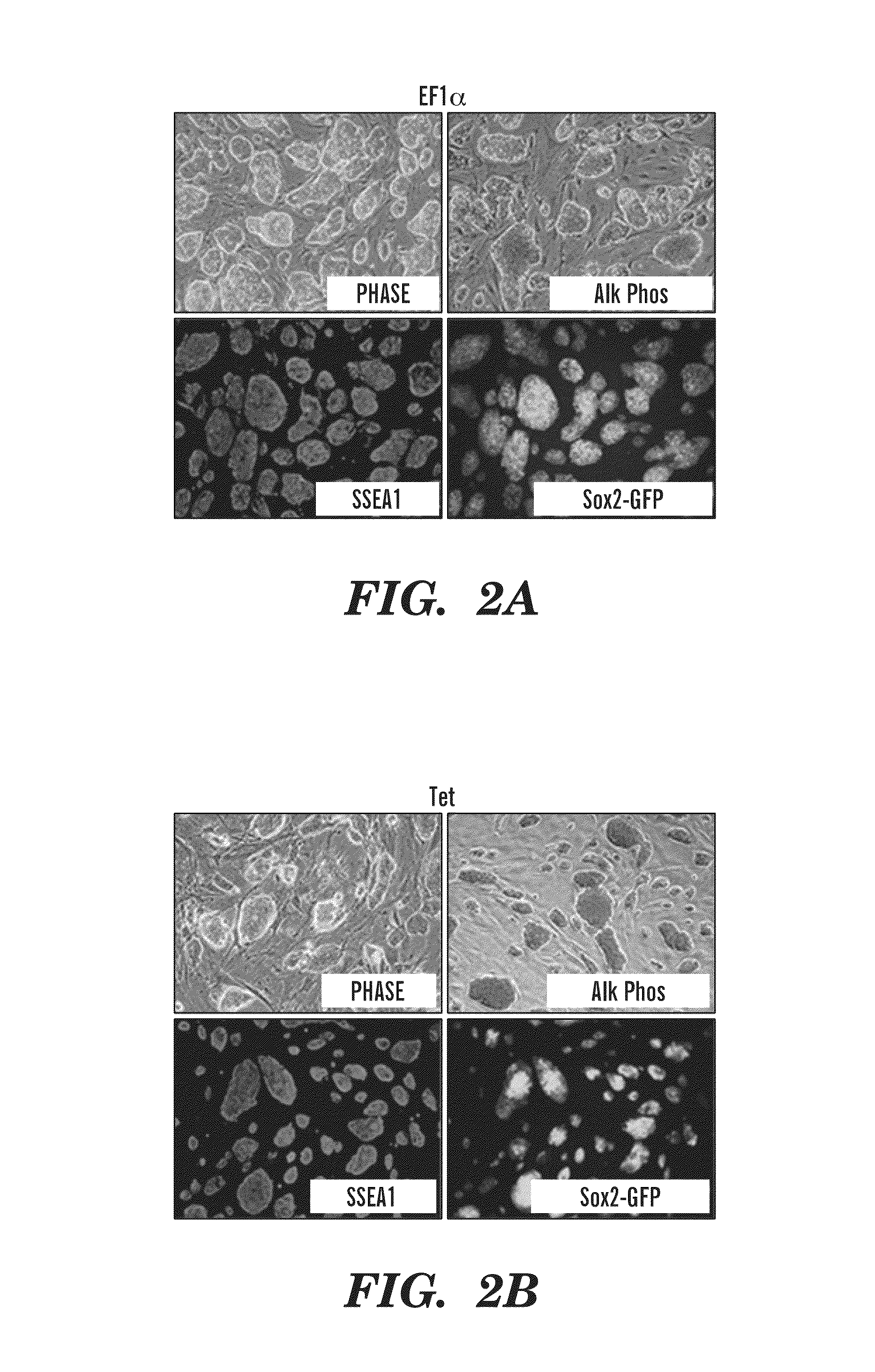
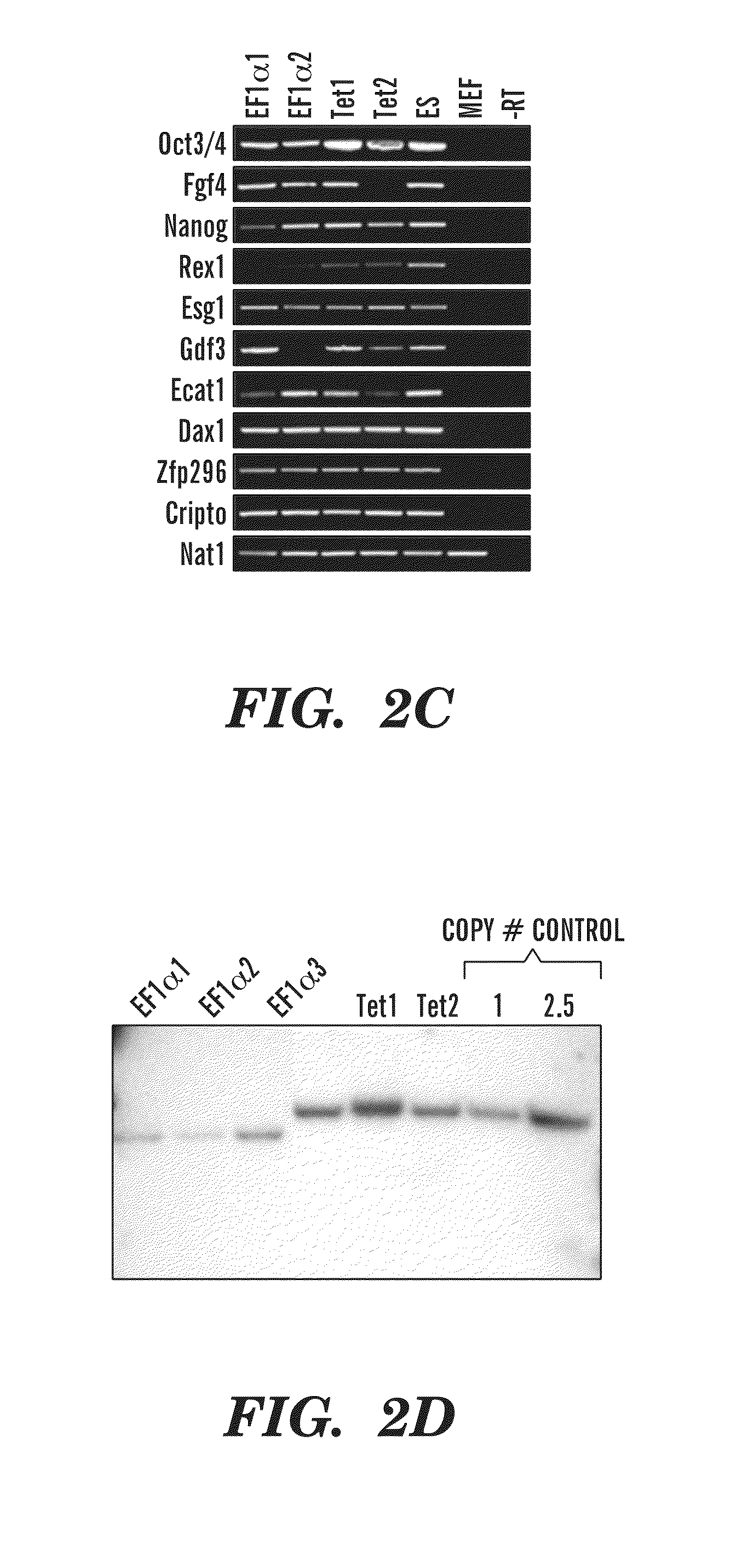
[0287]Previous studies have developed multicistronic lentiviral vectors based on a combination of an IRES element and 2A peptide sequences (Szymczak et al., 2004, Nat Biotechnol 22, 589-594) to express multiple genes simultaneously from a single lentiviral vector (Chinnasamy et al., 2006, Virol. J. 3:14). Using a similar approach a single lentiviral transfer gene plasmid was designed expressing a “STEM-Cell Cassette”, (hereafter pHAGE-STEMCCA). This cassette is comprised of a single multicistronic mRNA containing an IRES element separating two fusion cistrons. The two cistrons consist of Oct4 and Sox2 coding sequences fused to Klf4 and c-Myc, respectively, through the use of intervening sequences encoding ‘self-cleaving’ 2A peptides (FIG. 1A). Two forms of pHAGE-STEMCCA were generated, wherein the multicistronic transcript is driven by either a constitutive EF1α promoter or a doxycycline (dox)-inducible TetO-miniCM...
example 2
[0295]Reprogramming of somatic cells to a pluripotent state has been achieved by the introduction of four transcription factors, OCT4, KLF4, SOX2 and c-MYC using four independent retroviral vectors (Takahashi K. and Yamanaka S., Cell. 2006, 126:663-676). Following the reproduction and extension of these studies in both murine and human cells (Okita K, et al., Nature. 2007, 448:313-317; Maherali N, et al., Cell Stem Cell. 2007, 1:55-70; Wernig M, et al., Nature. 2007, 448:318-324; Takahashi K, et al., Cell. 2007, 131:861-872; Yu J, et al., Science. 2007; 318:1917-1920), it is now widely accepted that iPS cells share many of the characteristics of embryonic stem cells (ESC), including gene expression profiles, epigenetic signatures and pluripotency (Wernig M, et al., Nature. 2007, 448:318-324; Brambrink T, et al., Cell Stem Cell. 2008; 2:151-159; Meissner A, et al., Nature, 2008, 454:766-70; Mikkelsen T S, et al., Nature, 2007, 448:553-560; Stadtfeld M, et al., Cell Stem Cell. 2008, 2...
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap