Methods of Using Interleukin-10 for Treating Diseases and Disorders
a technology of interleukin-10 and disease, applied in the direction of immunological disorders, antibody medical ingredients, peptide/protein ingredients, etc., can solve the problems of life-threatening cytokine release syndrome, antigen-specific destruction of cancer, activation-induced cell death, etc., to prevent or limit activation-induced cell death, inhibit antigen presentation, and enhance the effect of activation-induced cell death
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
PEG-IL-10 Mediates CD8+ T Cell Immune Activation
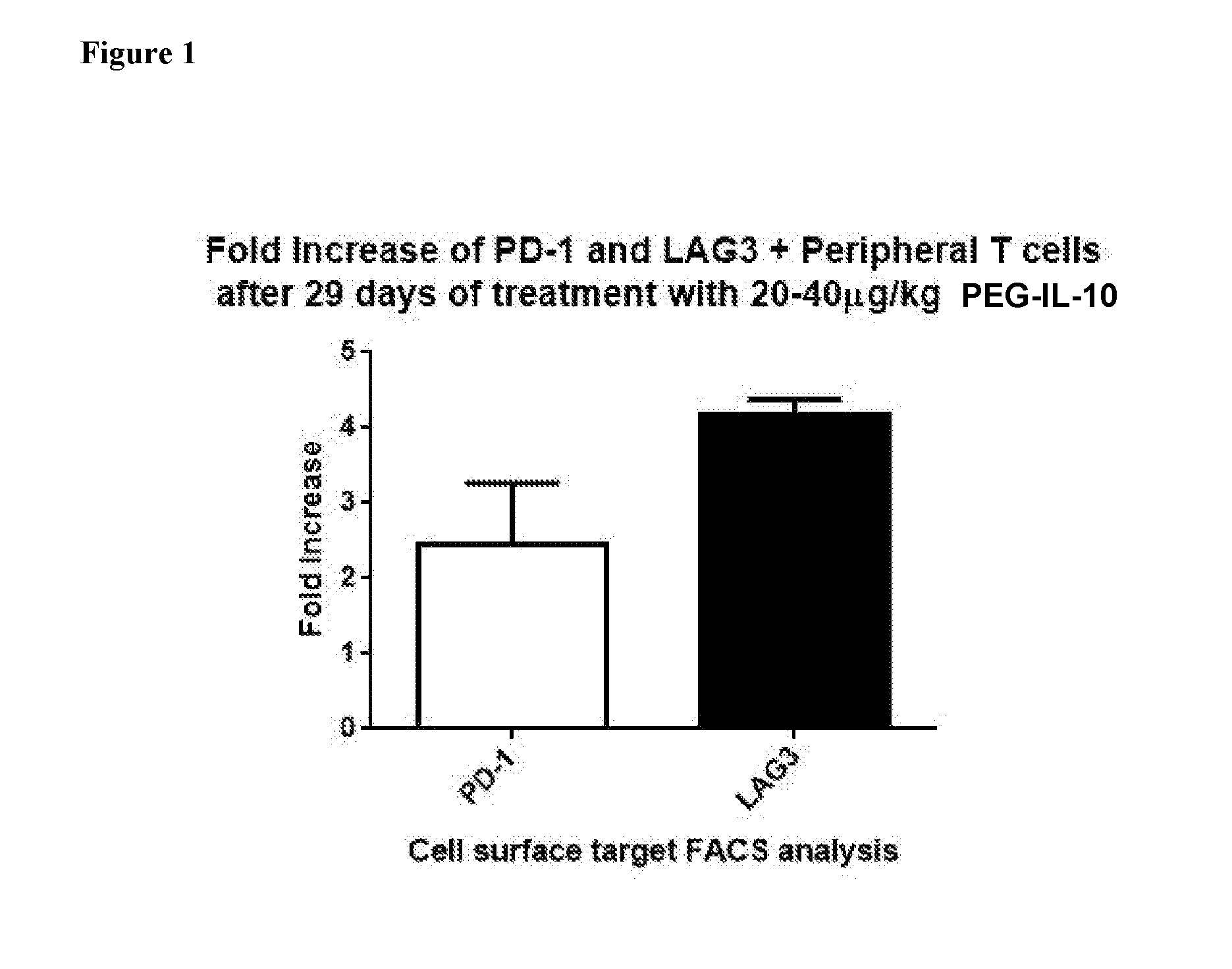
[0266]The change in the number of PD-1- and LAG3-expressing CD8+ T cells was determined in cancer patients before and after 29 days of treatment with PEG-rHuIL-10. Two patients who responded to the therapy with a sustained partial response had an increase of the PD1+CD8 T cells in the blood. The first patient (renal cell carcinoma) received 20 μg / kg PEG-rHuIL-10 SC daily and experienced a 71% reduction of total tumor burden after 22 weeks. The second patient (melanoma) received 40 μg / kg PEG-rHuIL-10 SC daily and experienced a 57% reduction of total tumor burden after 22 weeks.
[0267]Peripheral blood monocytic cells (PBMC) were isolated from the periphery of each patient pre-treatment and during the treatment period and were subjected to FACS analysis. As indicated in FIG. 1, the number of peripheral CD8+ T cells expressing PD-1 increased by ˜2-fold within 29 days and continued to increase during the treatment period., and the number of ...
example 2
PEG-IL-10 Enhances the Function of Activated Memory CD8+ T Cells
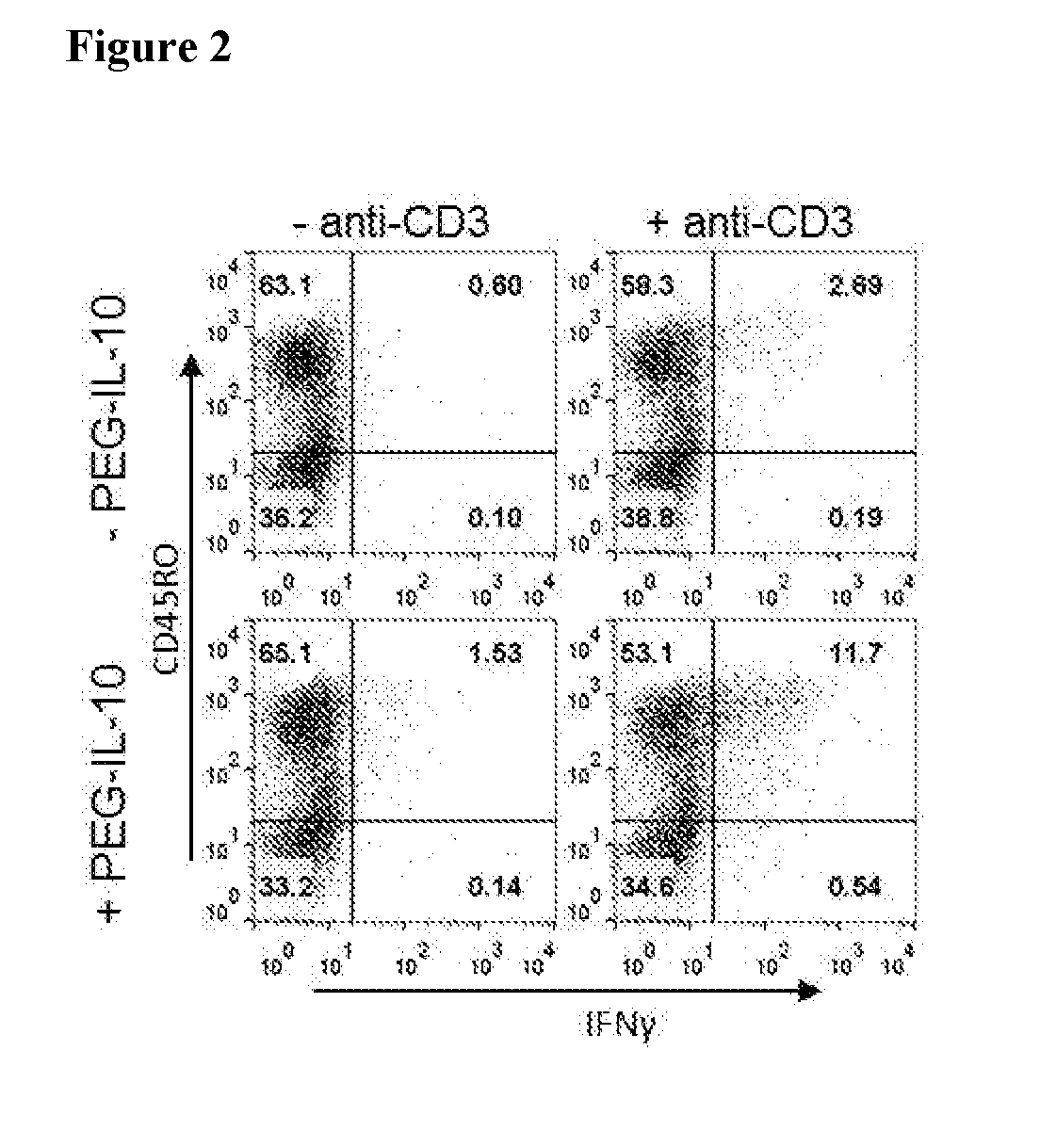
[0268]Memory T cells (also referred to as antigen-experienced T cells) are a subset of T lymphocytes (e.g., helper T cells (CD4+) and cytotoxic T cells (CD8+)) that have previously encountered and responded to their cognate antigen during prior infection, exposure to cancer, or previous vaccination. In contrast, naïve T cells have not encountered their cognate antigen within the periphery; they are commonly characterized by the absence of the activation markers CD25, CD44 or CD69, and the absence of memory CD45RO isoform. Memory T cells, which are generally CD45RO+, are able to reproduce and mount a faster and stronger immune response than naïve T cells.
[0269]Given that CAR-T T cells are derived from memory CD8+ T cells, the effect of PEG-IL-10 on memory CD8+ T cells was assessed in vitro using standard methodology, an example of which is described herein. As indicated in FIG. 2, PEG-IL-10 preferentially enhances IFNγ p...
example 3
PEG-IL-10 Treatment Results in a Greater Number of Activated Memory CD8+T Cells
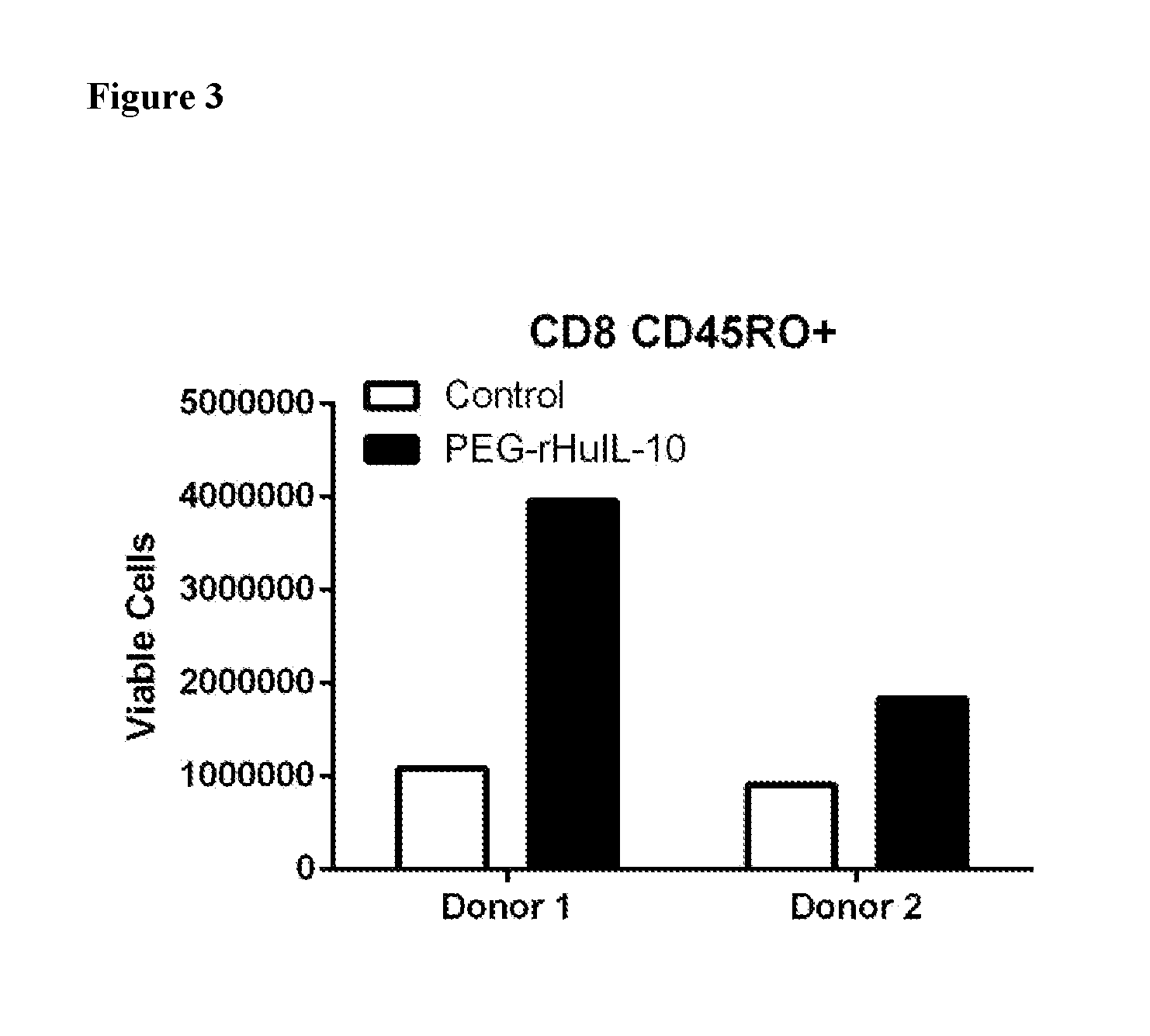
[0270]As described herein, CAR-T cell therapy is derived from memory CD8+ T cells. In order to be effective, infused memory CD8+ T cells must not only exhibit cytotoxicity, but must also persist (Curran K J, Brentjens R J. (20 Apr 2015) J Clin Oncol pii: JCO0.2014.60.3449; Berger et al., (Jan 2008) J Clin Invest 118(1):294-305). However, repeated activation of T cells leads to activation-induced cell death, which decreases the number of cells and thus the overall therapeutic efficacy.
[0271]Using the procedure described herein, the activation-induced cell death of human CD45RO+memory CD8+ T cells from two donors was determined with and without treatment with PEG-IL-10. As indicated in FIG. 3, treatment of human CD45RO+memory CD8+ T cells with PEG-IL-10 after two rounds of TCR and co-stimulation—induced activation resulted in a greater number of viable cells. These data indicate that PEG-IL-10 is capable of...
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentration | aaaaa | aaaaa |
| molecular mass | aaaaa | aaaaa |
| molecular mass | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap