Ubiquitin variant modulators of hect e3 ligases and their uses
a technology of hect e3 and variant modulators, which is applied in the field of ubiquitin variants, can solve the problems of affecting the development of selective modulators of hect e3s, affecting the potency and specificity of existing molecules, and affecting the effect of hect e3s
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
experimental examples
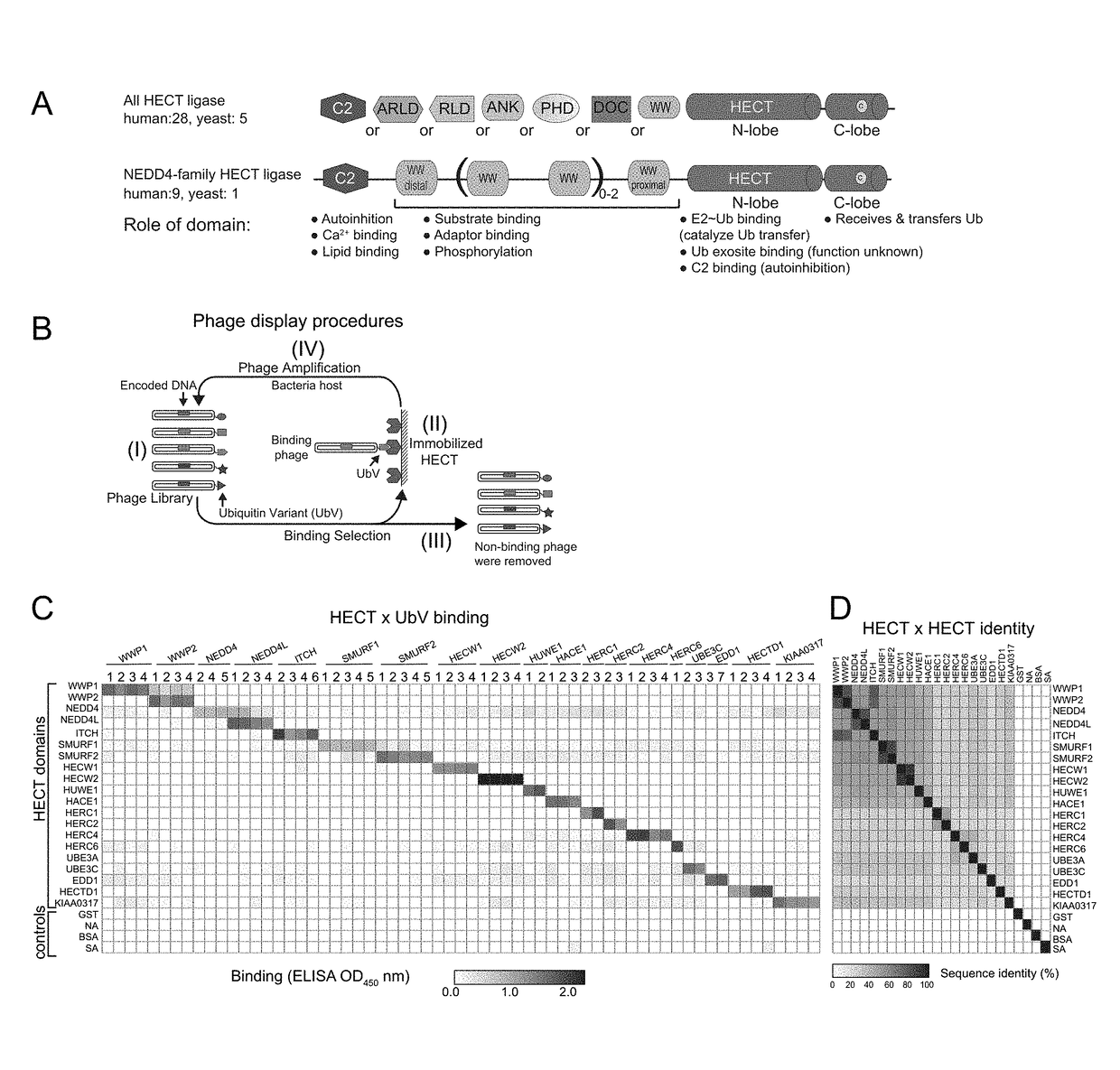
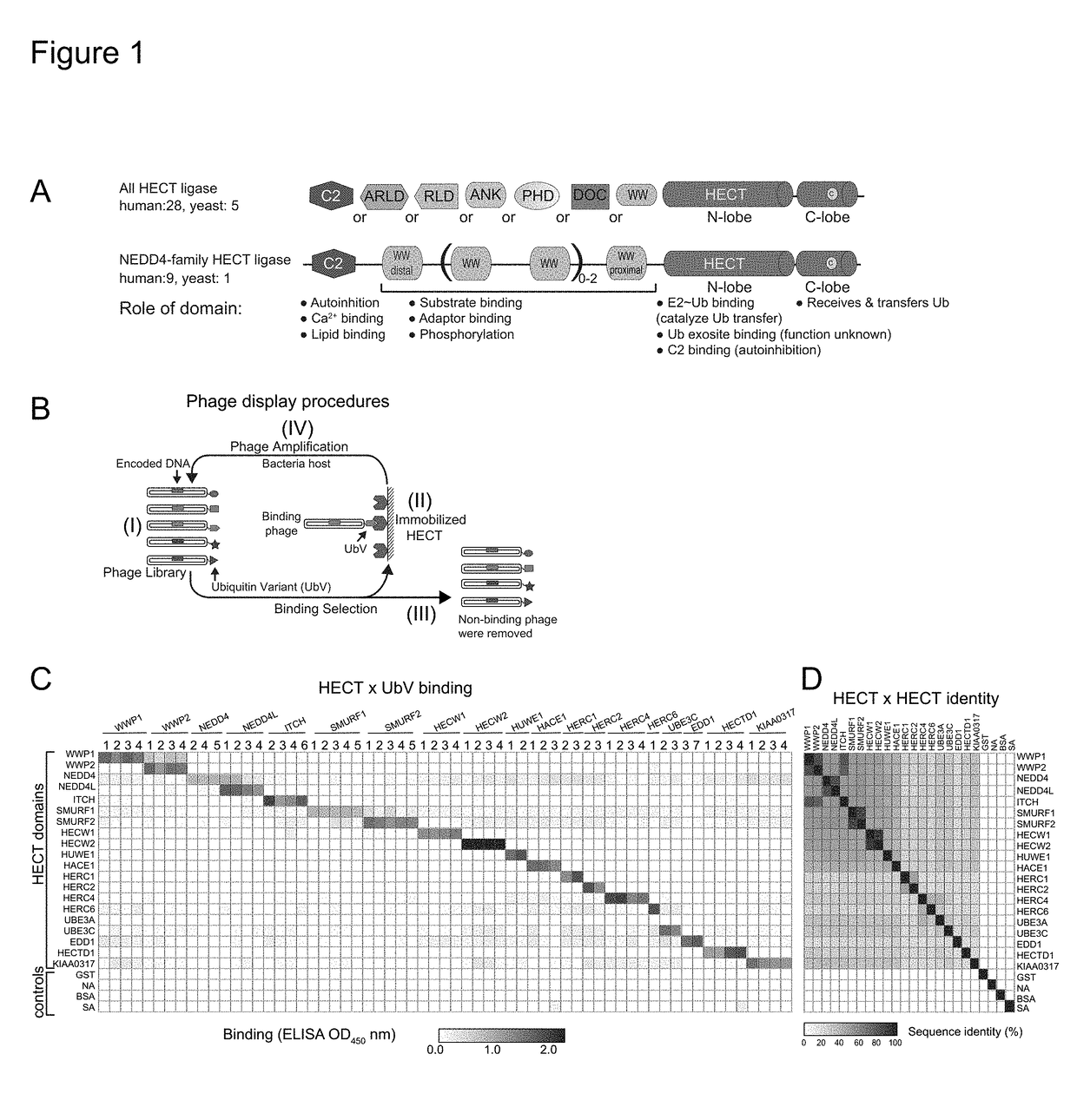
[0076]Development of potent and selective UbV modulators for 20 HECT E3 ligases We used a phage-displayed UbV library that varies almost all residues contacting the N-lobe exosite but only a subset of those mediating interactions in the transient catalytic intermediates. Binding selections (FIG. 1B) against purified HECT domains for 19 of 28 total human and 1 of 5 total yeast HECT E3s (Table 2) yielded 69 UbVs with a variety of substitutions across the binding surface (Table 3). Assessment of affinities for cognate HECT domains by measuring EC50 values (Table 4) confirmed higher affinity interactions for UbVs (in some cases EC5080% identity (Table 5).
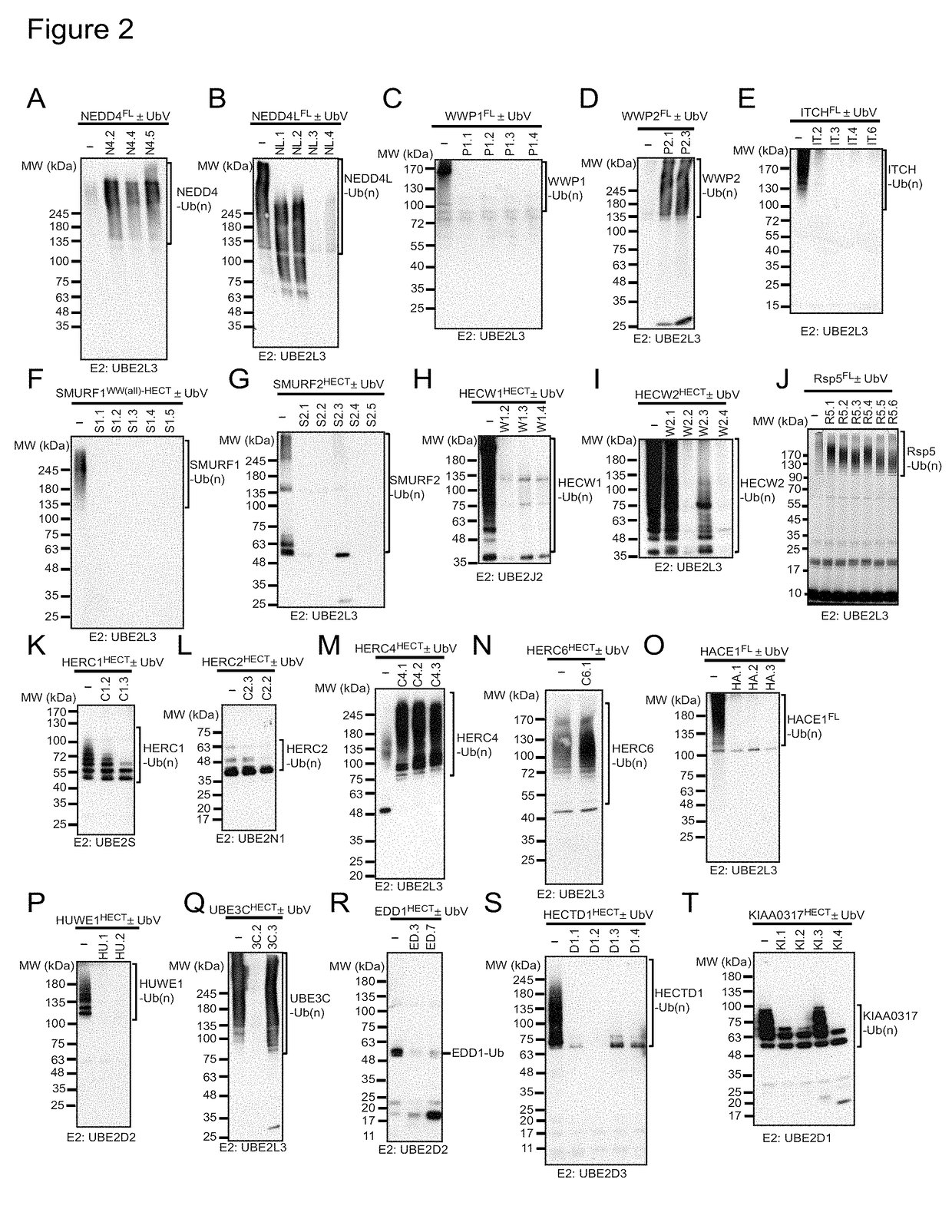
[0077]Whereas previous studies confirmed that DUB catalytic activity is potently inhibited by associated UbVs targeting their substrate-binding sites (Ernst et al., 2013; Phillips et al., 2013; Zhang et al., 2013), we hypothesized that UbVs targeting different sites on HECT E3s may modulate ligase activity in a variety of ways that migh...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Fraction | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com