Differential Identification of Pancreatic Cysts
a technology of pancreatic cysts and differential identification, applied in the field of diagnosis, can solve the problems of difficult to determine the type of cyst from conventional clinical, radiographic, or cytologic findings, and the management of these cysts is concomitantly becoming a major problem
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Materials and Methods
Patients and Specimens
[0048]The present study was approved by the Institutional Review Boards of Johns Hopkins Medical Institutions, Memorial Sloan Kettering Cancer Center, Wayne State University Emory University and the University of Indiana. Lesions were classified as IPMNs, MCNs, SPNs or SCAs using standard criteria (74). None of the patients with SCAs had clinical features of the VHL syndrome and all of the SPNs were Stage 1B, pT2N0M0 (74). IPMNs were subtyped by internationally accepted criteria (75).
[0049]Fresh-frozen tissue specimens of surgically resected cystic neoplasms of the pancreas were obtained through the prospectively maintained Johns Hopkins Surgical Pathology Tumor Bank and from collaborating institutions (Memorial Sloan Kettering Cancer Center, Emory University, the University of Utrecht and Wayne State University). IPMNs, SCAs, SPNs and two MCNs specimens were microdissected using a razor blade. In these cases, serial frozen sections were us...
example 2
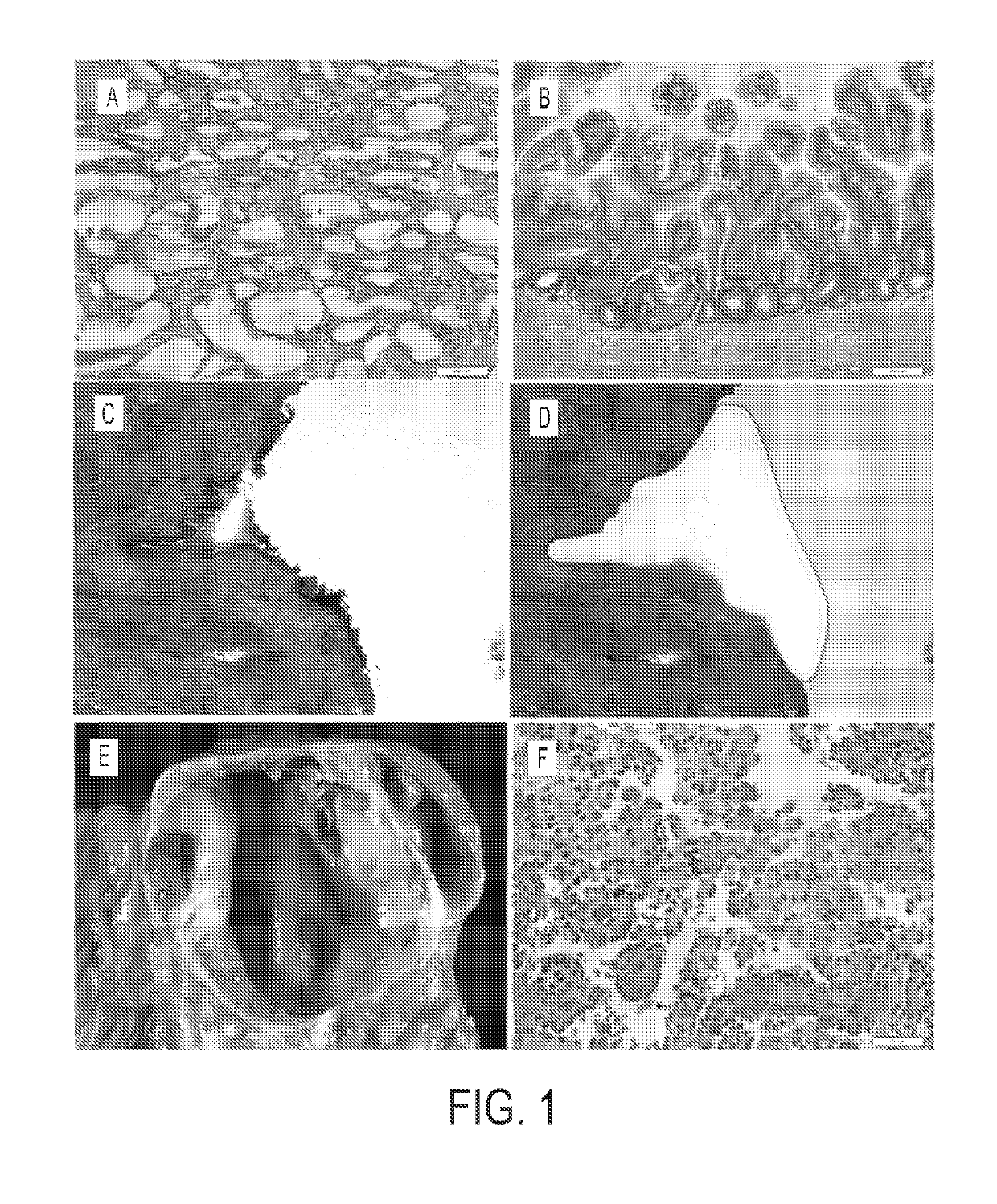
[0061]Experimental design. Neoplastic cysts are composed of a mixture of neoplastic epithelial cells and non-neoplastic cells (stromal, vascular, and inflammatory (17)) (FIG. 1). To maximize our ability to detect mutations, we carefully microdissected the neoplastic epithelial cells from the non-neoplastic cells. This was most difficult in the MCNs because of the cellular ovarian-type stroma present in these lesions (FIG. 1C, D). Following microdissection, the neoplastic cell content of each of the cyst samples analyzed in this study was at least 33%.
[0062]DNA from 32 microdissected cysts, eight of each of the four types, as well as matched DNA from normal tissues of the same patients, was used in this study. The clinical and histopathologic characteristics of the patients and their cystic lesions are detailed in Table S 1. The DNA was ligated to adapters and amplified using standard Illumina protocols. The amplified DNA was then captured with a 50 MB SureSelect Enrichment System. T...
example 3
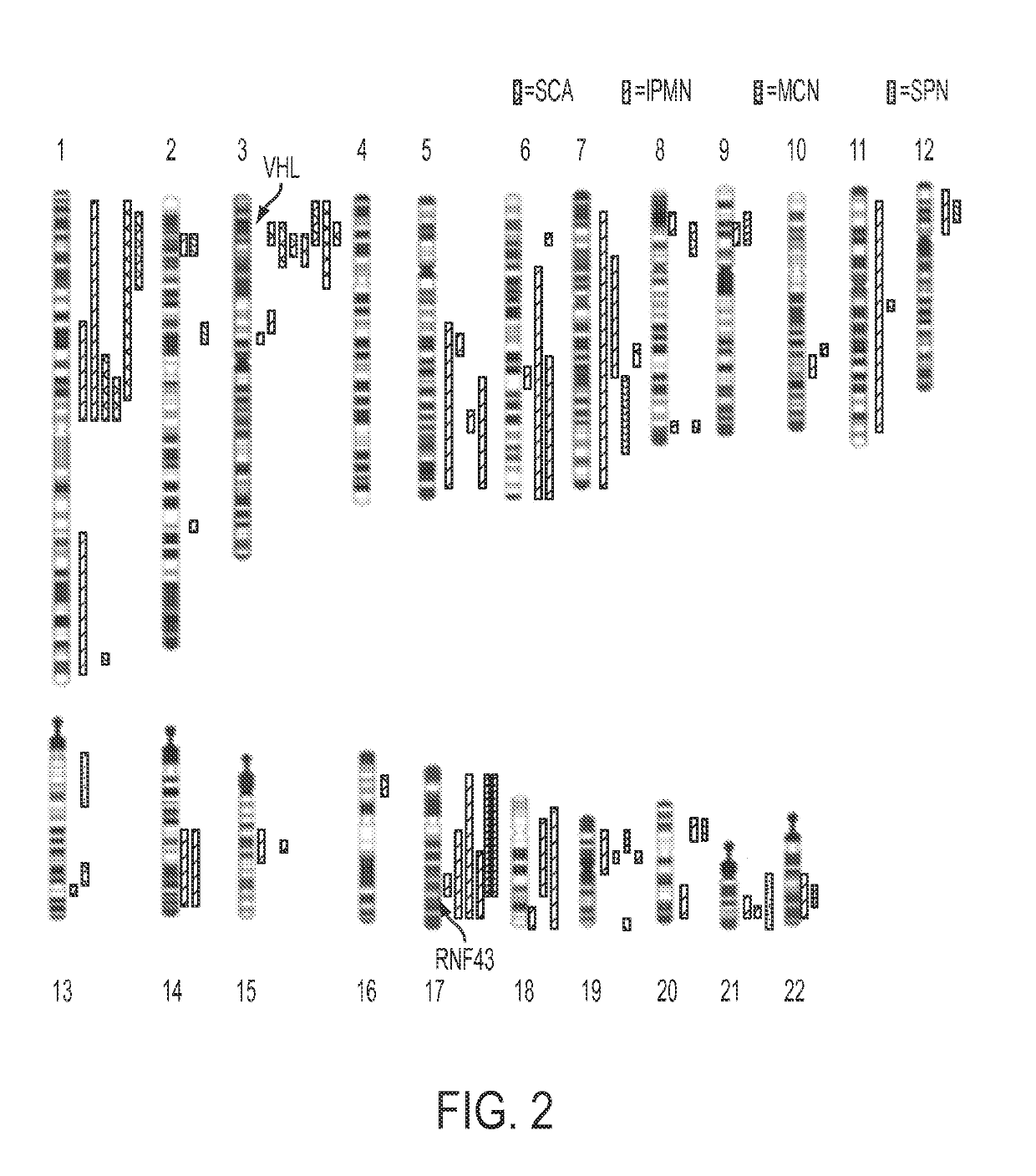
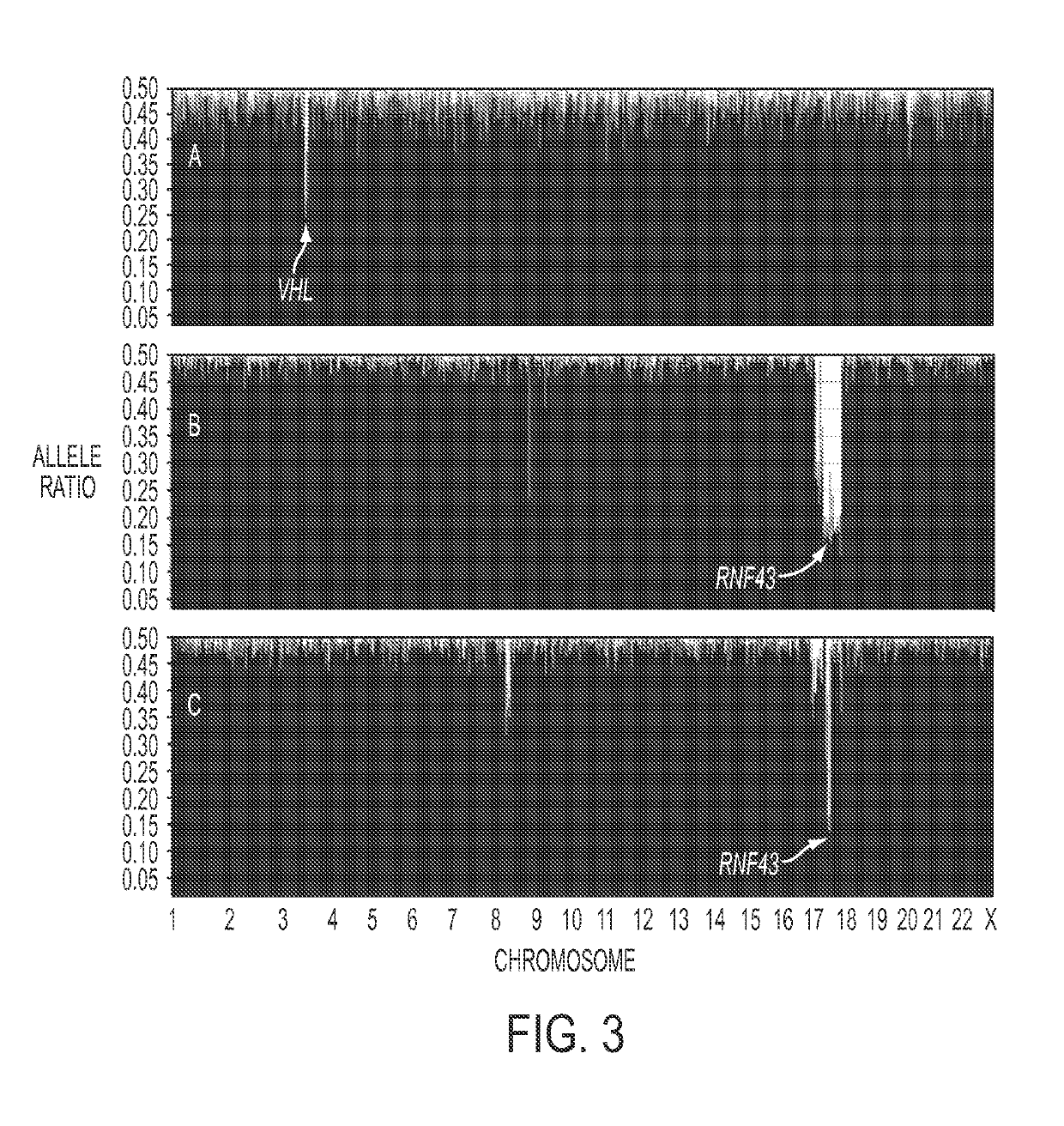
[0063]Analysis of SCAs. Using single nucleotide polymorphisms in the sequences captured by the SureSelect Enrichment System, we were able to identify 15,190±428 heterozygous variants in the matched normal DNA samples of the eight SCA patients. Loss of heterozygosity (LOH) of at least one chromosomal region was identified in each of the eight SCAs studied (FIG. 2, Table S3). The maximum degree of LOH (70%±13%) confirmed the high fraction of neoplastic cell content achieved upon microdissection. The only region that was lost in the majority of SCAs was on chromosome 3p (FIG. 2, example in FIG. 3A). Seven of the eight SCAs lost chromosome 3p alleles, with the losses demarcated by bases 9,934,713 to 12,850,443. To determine whether this LOH was associated with reduplication of the remaining allele, we compared the copy number of all sequences lying within the regions of LOH to those of all other chromosomal regions in the same cysts. This was accomplished by comparing normalized tag cou...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com