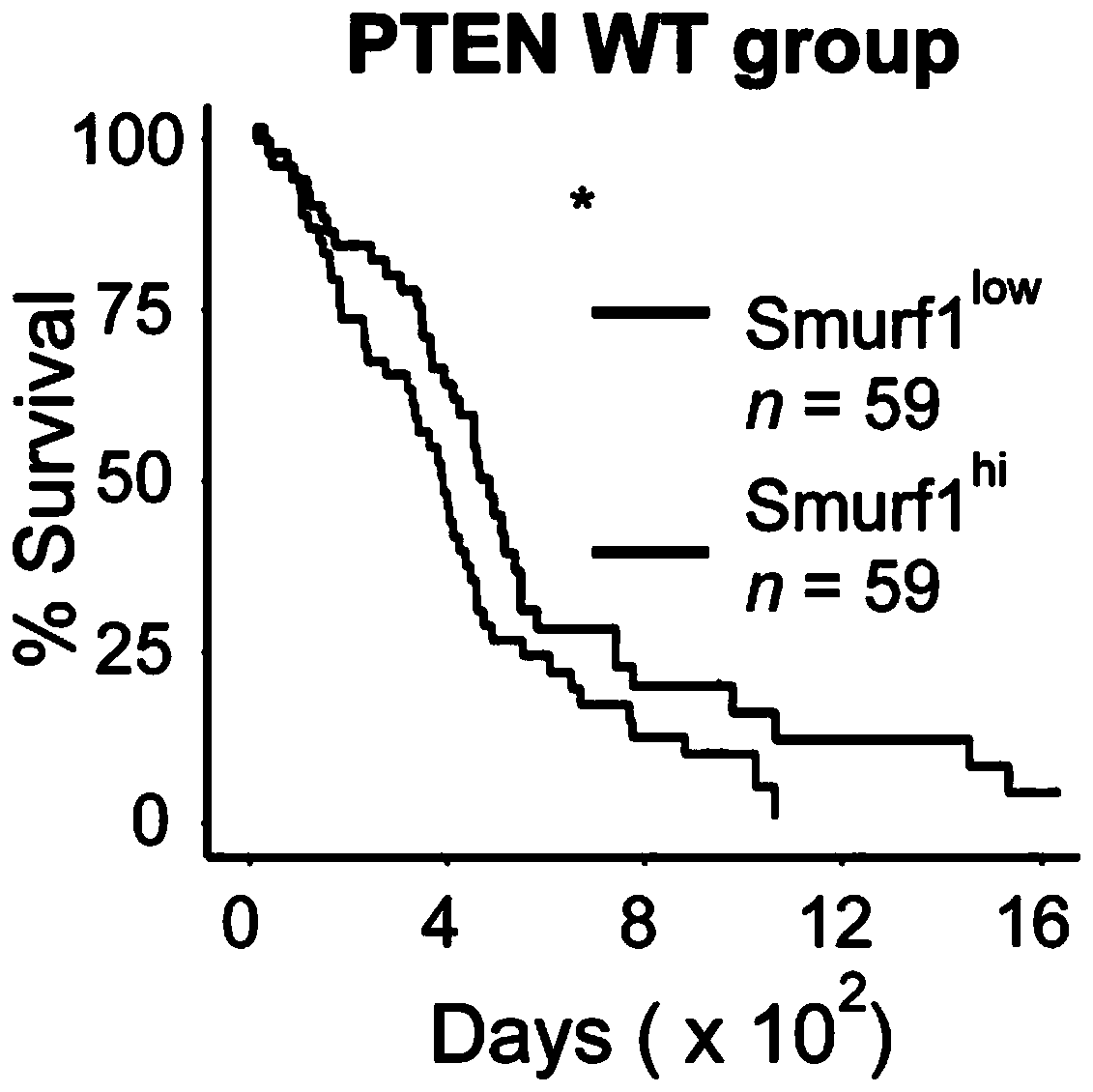
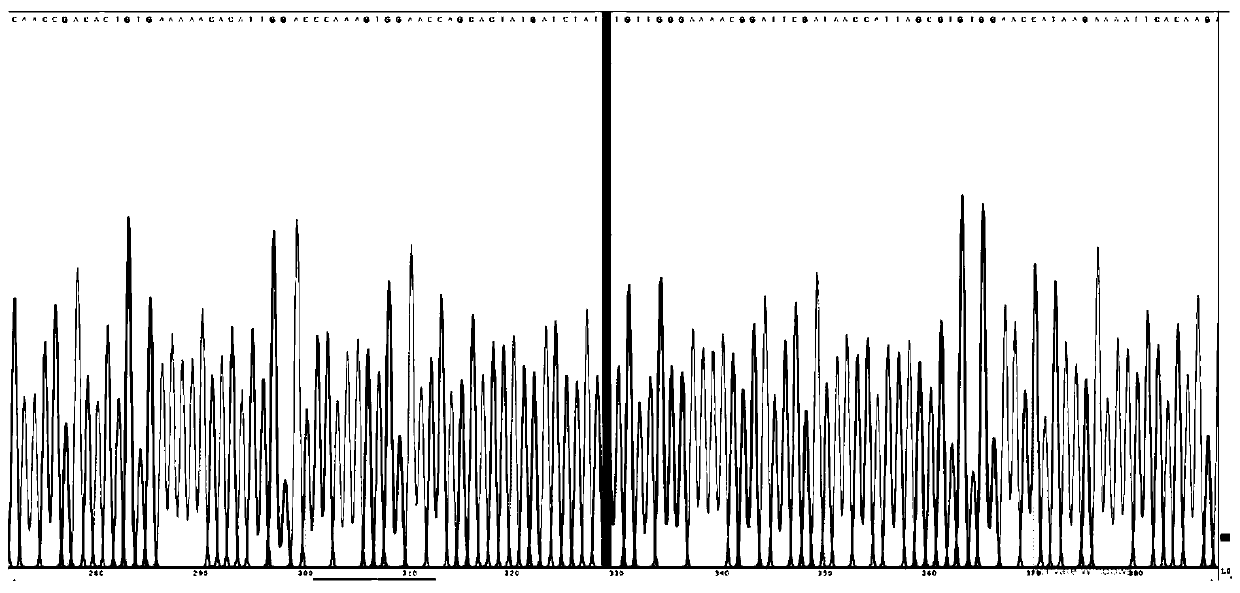
Ubiquitin ligase Smurf1 mutant, coding gene and application
A ubiquitin ligase, mutant technology, applied in the fields of molecular biology and medicine, can solve the problem of unclear molecular mechanism, and achieve the effect of inhibiting the occurrence of cancer
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] Embodiment 1: expression vector construction
[0027] 1. Experimental method
[0028] Myc-Smurf1 plasmid, pET-21a plasmid, HA-Ub plasmid, lentiviral expression vector PCDH-3HA, lentiviral packaging plasmid PSPAX2 and envelope plasmid PMD2G were purchased from Addgene.
[0029] (1) Site-directed mutation (taking the 69th amino acid mutation "Y69F" from Y to F as an example, the construction process of other types of mutations is similar)
[0030] The Myc-Smurf1 plasmid (other plasmids inserted with Smurf1 can also be used) was subjected to site-directed mutagenesis. The amino acid sequence of Smurf1 is shown in SEQ ID No.1, and its nucleotide sequence is shown in SEQ ID No.4.
[0031] The specific operation is as follows:
[0032] The primers for site-directed mutagenesis are listed in the table below:
[0033] Primer name Primer sequence (5'-3') SEQ ID XQ5 CACTATGATCTATTTGTTGGGAAA ACG SEQ ID No: 5 XQ6 CTGGTTCCACTTTGGGTCCAA SEQ ID No: ...
Embodiment 2
[0051] Example 2: Protein expression and functional identification
[0052] 1. Experimental method
[0053] (1) Recombinant lentiviral packaging
[0054] The empty vector, wild-type and mutant recombinant lentiviral expression vectors were transfected into 293T cells by vigo using PSPAX2 and PMD2G plasmids respectively, the medium was changed 4 hours after transfection, and the virus liquid was collected 72 hours after the medium change (supernatant of transfected 293T cells solution), centrifuged at 3000rpm for 5min, filtered through a 0.45μm membrane filter, and stored at -80℃ after aliquoting.
[0055] 293T cells were cultured in DMEM (Dulbecco's modified Eagle medium) (Gibco, Grand Island, NY, USA) containing 10% fetal bovine serum (fetal bovine serum, FBS) (Gibco, Grand Island, NY, USA), and the culture medium contained 100 IU / ml penicillin and 100 μg / ml streptomycin (Gibco). Cells were placed at 37°C 5% CO 2 cultured in an incubator.
[0056] (2) Lentivirus infecti...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com