A small molecule inhibitor of polymyxin-resistant protein and its application
A small molecule inhibitor, polymyxin technology, applied in the field of biomedicine, can solve the problem of reducing the efficacy of polymyxin, achieve the effect of inhibiting protein activity and effectively applying space
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
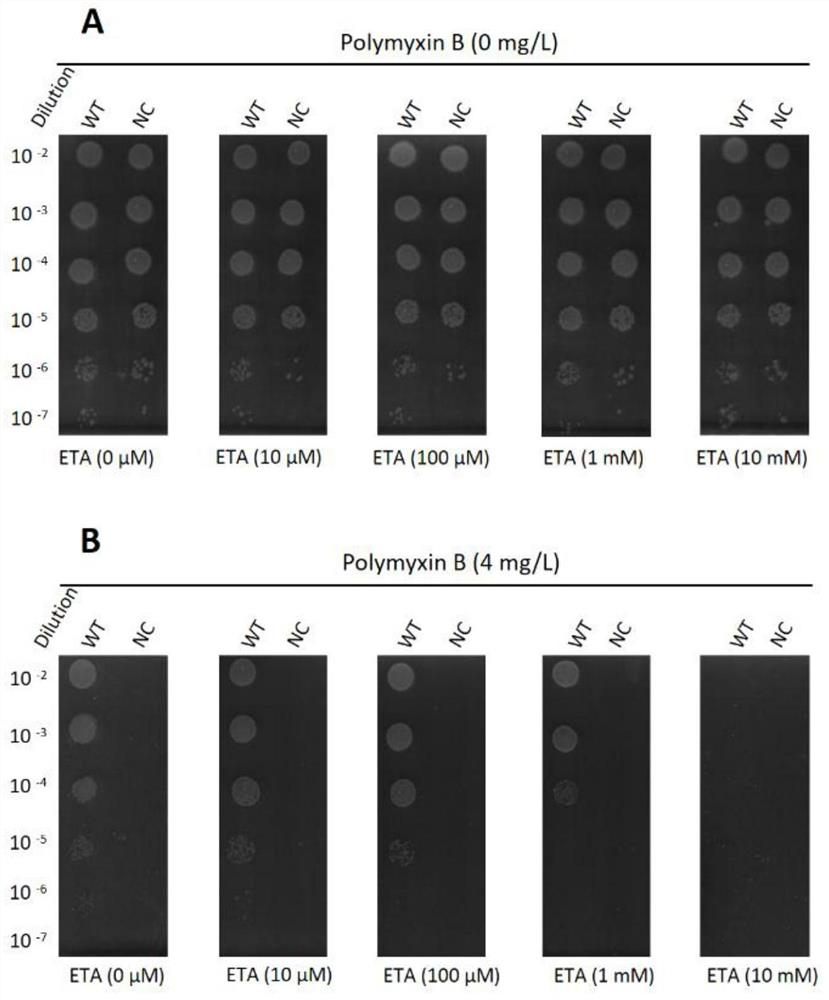
[0023] Detect the inhibitory effect of ethanolamine small molecule inhibitors on MCR-1 positive bacteria, comprising the following steps:
[0024] 1) According to the experimental design, first add different concentrations of polymyxin B (polymyxin B) into the MH (Muller Hinton) agar that has been heated and dissolved, and equilibrated in a water bath at 45-50°C, and then the diluted Small molecule inhibitor ethanolamine (ETA) solutions of different concentrations were added to the above-mentioned agar, mixed evenly and poured into a sterilized plate, the thickness of the agar was 3-4 mm;
[0025] 2) Prepare MCR-1-positive bacteria (wide type, WT) and MCR-1-negative bacteria (nagtive cotrol, NC) bacterial suspensions with concentrations equivalent to 0.5 McFarland standard turbidimetric tubes, and serially dilute to 10% of the mother liquor. -2 , 10 -3 ... 10 -7 concentration;
[0026] 3) Inoculate the diluted bacterial solution onto the MH agar plate in step 1) in sequence...
Embodiment 2
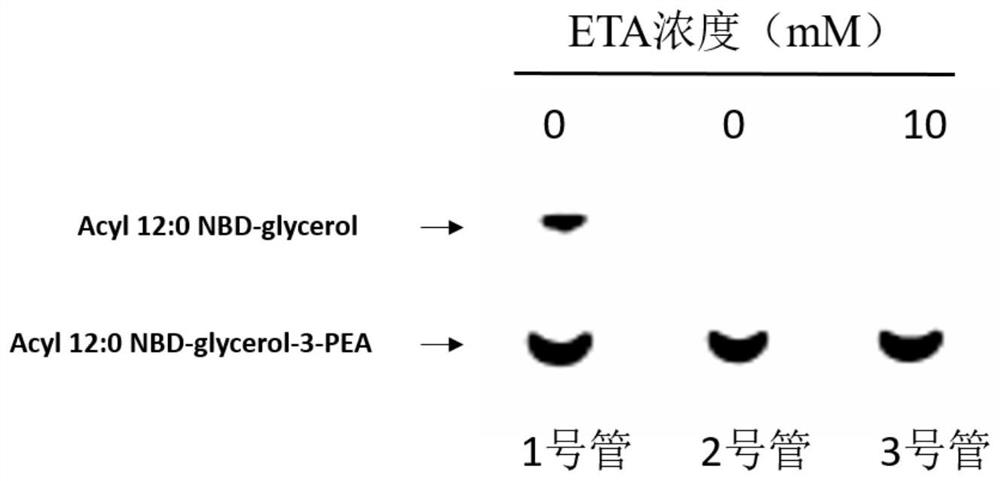
[0029] Detecting the inhibitory effect of small molecule inhibitors on MCR-1 protein activity, comprising the following steps:
[0030] 1) Construction of prokaryotic expression plasmid for MCR-1 protein: the gene sequence of MCR-1 was inserted into pET28a plasmid through NcoI and XhoI restriction sites to construct pET28a-MCR plasmid, which was verified by DNA sequencing; MCR-1 The gene sequence is shown in SEQID NO.1;
[0031] 2) Expression of MCR-1 protein: pass the above pET28a-MCR plasmid through CaCl 2 coli Roseta strain, screened by kanamycin and chloramphenicol, the strains grown on LB (Luria-Bertani) culture plates containing kanamycin and chloramphenicol were inoculated into In the LB liquid medium of kanamycin and chloramphenicol, culture at 37°C, 220rpm to the logarithmic growth phase, then add IPTG (isopropylthiogalactopyranoside) to 0.5mM, and induce at 20°C for 20 hours. Finally, the bacteria were collected by centrifugation;
[0032] 3) Purification of MCR-1...
Embodiment 3
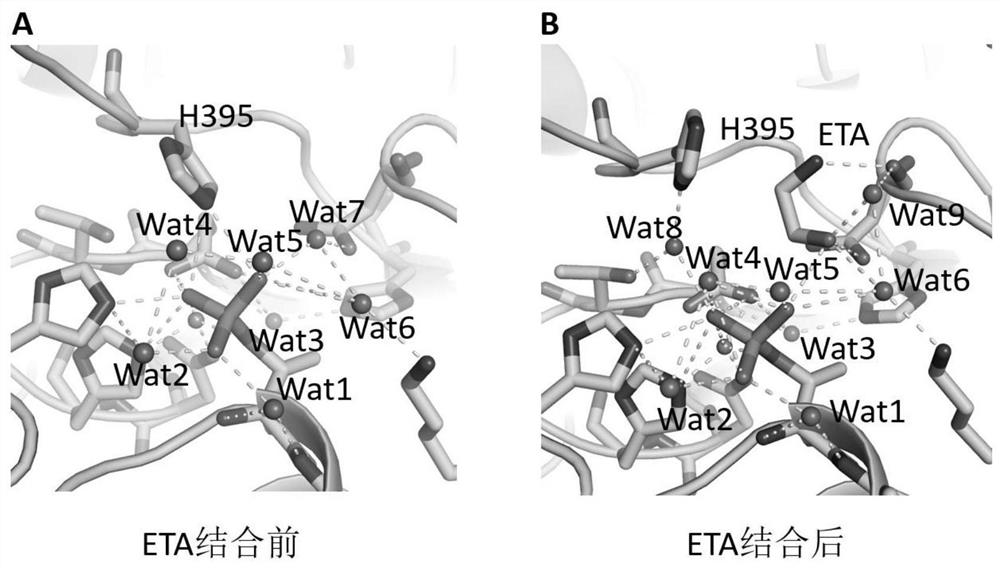
[0038] The inhibitory effect of the MCR-1 inhibition site is detected by the method of determining the polymyxin minimum inhibitory concentration of the mutant strain, comprising the following steps:
[0039] 1) Using the pET28a-MCR plasmid obtained in Example 2, 8 possible MCR-1 inhibitory sites: the 246th glutamic acid (E246), the 285th threonine (T285), the 329th day Paragine (N329), Lysine 333 (K333), Histidine 395 (H395), Aspartic acid 465 (D465), Histidine 466 (H466) and 478 Histidine (H478) was subjected to site-directed mutation and mutated into alanine, respectively.
[0040] 2) Pass the above 8 kinds of mutated pET28a-MCR plasmids through CaCl 2 Transformed into Escherichia coli Roseta strain, screened by kanamycin and chloramphenicol, and verified by DNA sequencing.
[0041] 3) Add different concentrations of polymyxin B (polymyxin B) to the MH (Muller Hinton) agar that has been heated and dissolved, and equilibrated in a water bath at 45-50°C, mix well and pour i...
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com