Pharmaceutical composition containing polymyxin and application thereof
A technology of polymyxin and composition, applied in the field of pharmaceutical compositions including polymyxin and its synergist, can solve the problem of difficulty in meeting demand, low success rate, long development cycle of new antibiotic drugs, etc. problem, to achieve good therapeutic effect, increase bioavailability, and reduce the effect of drug dosage
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0042] The present invention provides a pharmaceutical composition containing polymyxin, said pharmaceutical composition comprising C507-0440 and polymyxin, wherein the chemical name of C507-0440 is N-[3-(methylthio)phenyl] -2,3-Dihydro-1H-cyclopenta[b]quinolin-9-amine, the PubChem SID number is 328424803, the specific structural formula is as follows:
[0043]
[0044] The pharmaceutical composition is the combination of C507-0440 or its pharmaceutically acceptable salt and polymyxin, or the mixed preparation of C507-0440 or its pharmaceutically acceptable salt and polymyxin.
[0045] Wherein, the polymyxin is polymyxin A, polymyxin B, polymyxin C, polymyxin D or polymyxin E.
[0046] Among them, polymyxin B is preferred in the pharmaceutical composition, and the concentration of polymyxin B is 0.023-64 ug / mL.
[0047] Wherein, the mass ratio of C507-0440 or its pharmaceutically acceptable salt to polymyxin B is 0.22-5300:1.
[0048] Wherein, in the pharmaceutically acce...
Embodiment 2
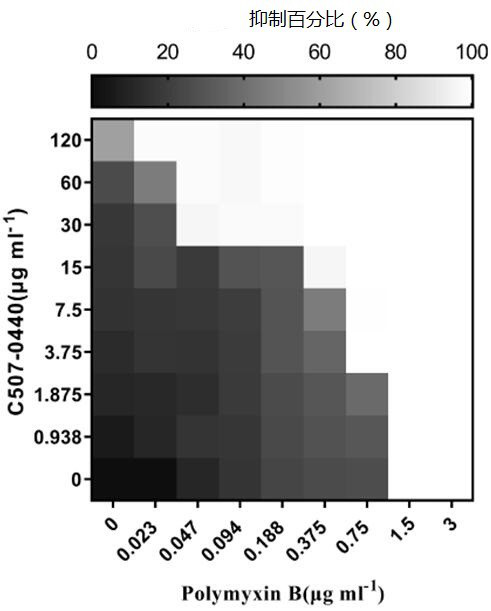
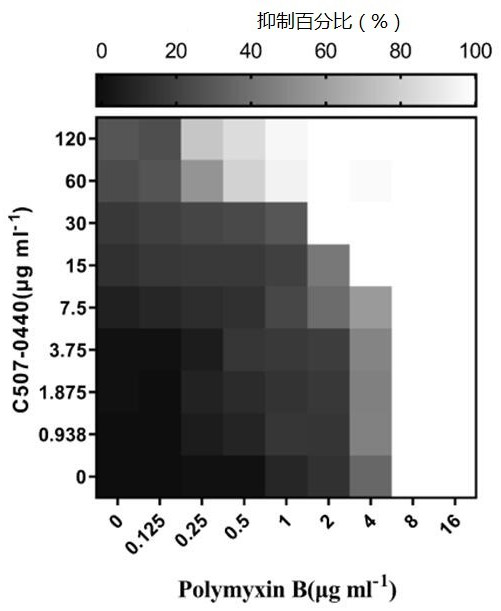
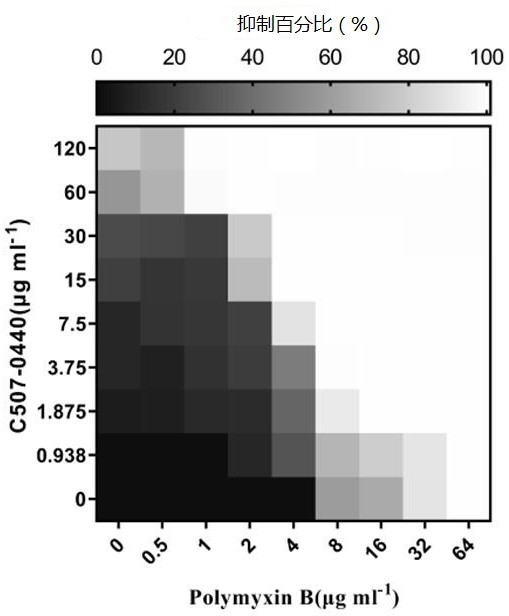
[0051] In this example, Klebsiella pneumoniae standard strain ATCC13883, Klebsiella pneumoniae clinical drug-resistant strain SZ021, Klebsiella pneumoniae clinical drug-resistant strain SZ206, Klebsiella pneumoniae clinical drug-resistant strain SZ301, Bao Acinetobacter mannequin ATCC19606 standard strain, Acinetobacter baumannii clinical drug-resistant strain SZ362, Pseudomonas aeruginosa model strain PA14 and Escherichia coli standard strain ATCC25922 were shaken at 37°C and 220 rpm until OD 600 =0.6~0.8, the combined effect of C507-0440 and polymyxin B was determined by checkerboard experiment, so as to further illustrate the present invention. The specific steps of the checkerboard experiment are as follows:
[0052] A. OD 600 = 0.6 ~ 0.8 of the bacterial culture density adjusted to OD 600 =0.001;
[0053] B. Polymyxin B was serially diluted 2 times along the abscissa, and C507-0440 was serially diluted 2 times along the ordinate;
[0054] C. Set up a blank group (a...
Embodiment 3
[0058] In this example, the mechanism by which C507-0440 enhances the activity of polymyxin B in Klebsiella pneumoniae is explored through proteome experiments, so as to further illustrate the present invention.
[0059] Control group: Klebsiella pneumoniae standard strain (ATCC13883) was inoculated into fresh LB broth medium supplemented with dimethyl sulfoxide at a volume ratio of 1:100, and cultured with shaking at 37°C and 220 rpm until OD 600 =0.6~0.8, 10000rpm, 3min centrifuge to collect the bacteria, then wash twice with pre-cooled PBS; resuspend the bacteria pellet, resuspend with four times the volume of lysis buffer, and sonicate on ice, at 15000rpm, 4℃ Centrifuge for 10 minutes, collect the supernatant, trypsinize, HPLC fractionation, and perform mass spectrometry analysis;
[0060] Single-drug group: inoculate the Klebsiella pneumoniae standard strain (ATCC13883) at a volume ratio of 1:100 into fresh LB broth medium supplemented with 3.175ug / mL C507-0440, at 37°C, ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com