A kind of antitumor compound and its preparation method and application
A compound and anti-tumor technology, applied in anti-tumor drugs, medical preparations containing active ingredients, organic chemistry, etc., can solve the problems of limited drug application, no small molecule compounds, etc., and achieve high yield, good effect, Effects of Good Medicinal Prospects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037] Example 1 Preparation of TI17
[0038] The synthetic reaction formula is as follows:
[0039]
[0040] The specific preparation method is:
[0041] Compound 1 was prepared according to the method of patent US2006 / 160803.
[0042] Compound 1 (1.84g, 10mmol) and compound 3 (1.90g, 10mmol) were dissolved in DMF (20mL) and stirred at room temperature for 12 hours. The solvent was evaporated to dryness under reduced pressure, and the residue was recrystallized from ethyl acetate / n-hexane (2:1) to obtain the target compound 2 (2.62 g, yield 70.0%) as a white solid. LC-MS:375[M+1] + ,749[2M+1] + ; 1 H NMR (400MHz, DMSO-d 6 )δ11.60(brs,1H),9.75(s,1H),8.38(d,J=6.0Hz,2H),7.41(d,J=8.4Hz,2H),7.15(d,J=5.6Hz, 2H), 7.08(d, J=8.8Hz, 2H), 5.75(t, J=6.8Hz, 1H), 5.62(t, J=6.8Hz, 1H), 3.84(s, 2H), 3.11(dd, J 1 =2.0Hz,J 2 =10.4Hz,1H),2.92-2.97(m,2H),2.88(dd,J 1 =2.0Hz,J 2 =10.4Hz,1H),0.92-2.93(m,2H),0.01-0.03(m,2H).
Embodiment 2
[0043] Example 2 Molecular docking of TI17 and TRIP13
[0044] 1. Method
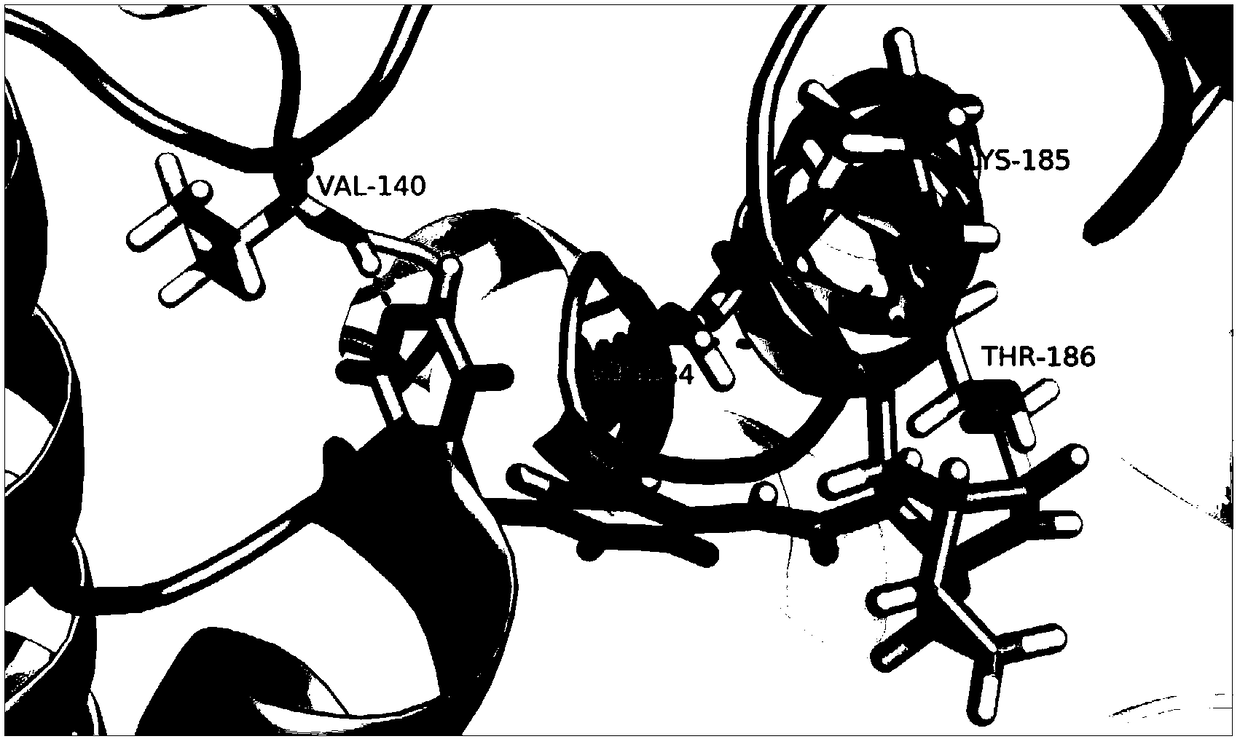
[0045] The C. elegans TRIP13 crystal structure (PDB number: 4XGU) was downloaded from the protein crystal structure database PDB, and used as a template to obtain the three-dimensional structure of human TRIP13 protein by homology modeling (Schrodinger2010). Then the compound small molecule TI17 was docked onto TRIP13 (Glide5.6, docking accuracy: XP).
[0046] 2. Results
[0047] see results figure 1 .
[0048] Conclusion: TI17 is well docked with TRIP13 protein, and it is a compound targeting TRIP13 gene.
Embodiment 3
[0049] Example 3 TRIP13 protein inhibitory activity of TI17
[0050] 1. Experimental materials
[0051] (1) Main reagents: TI17 (synthesized according to the method of Example 1); TRIP13 protein (synthesized by Shanghai Institute of Materia Medica, Chinese Academy of Sciences).
[0052] (2) Main instrument: Bruker AvanceⅢ-600 nuclear magnetic resonance wave spectrum instrument.
[0053] 2. Experimental method
[0054] (1) Sample preparation: the sample was dissolved in phosphate buffer (20mM NaPO 4 , 100 mM NaCl, 2% DMSO), DMSO was used as an internal reference.
[0055] (2) NMR tube positioning.
[0056] (3) On-machine operation: the sampling temperature is 25°C.
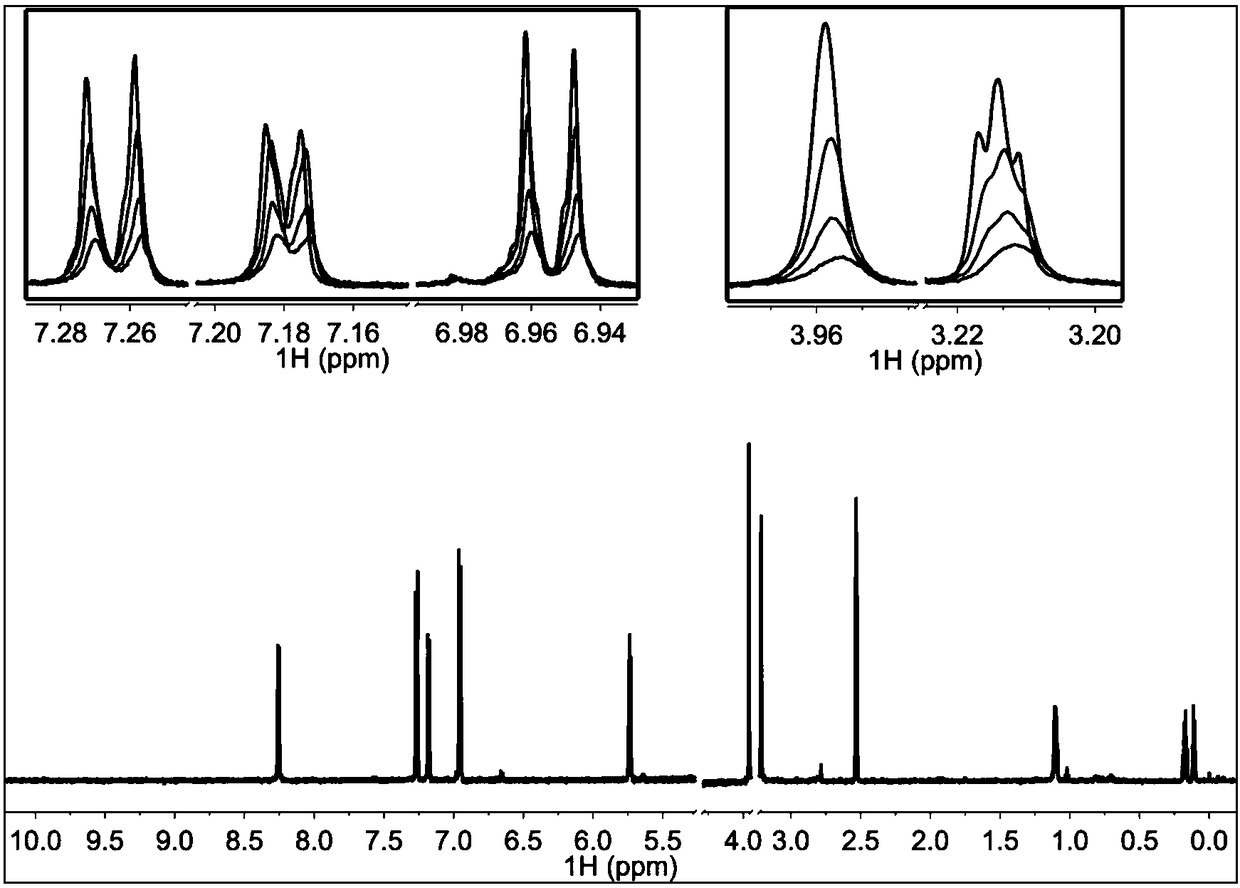
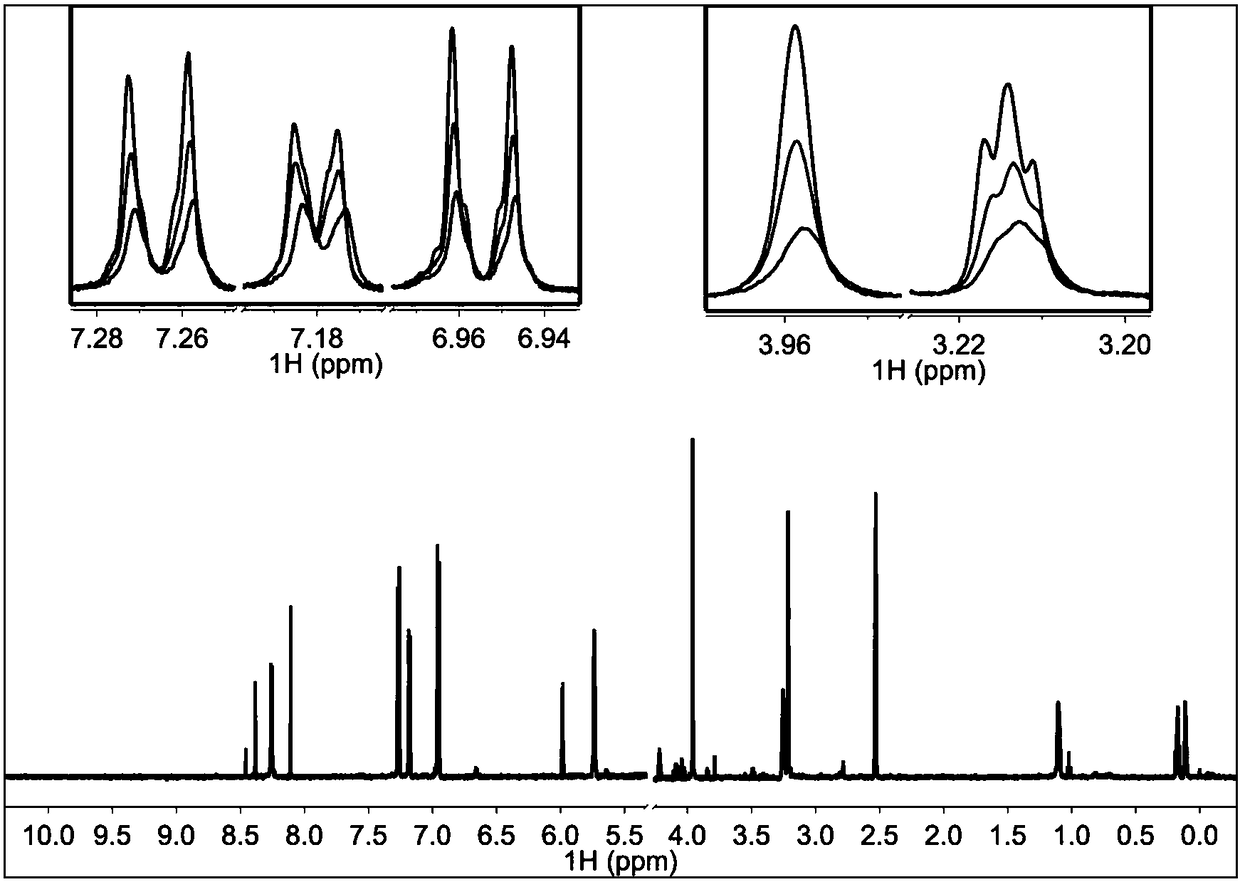
[0057] 3. Experimental results
[0058] figure 2 In 200μM TI17 solution alone, NMR Pop is red, when the molar ratio of TI17 and TRIP13 protein is 40:1, Pop is green, when the molar ratio is 20:1, Pop is blue-green, and the molar ratio is 10 :1, Pop is blue. This figure shows that the binding of TI17 to TR...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com