Systems, methods, and devices for sterilizing antiseptic solutions
a technology of antiseptic solutions and sterilization methods, applied in the field of sterilization, can solve the problems of reducing the overall concentration of active drug molecules, reducing the antiseptic effect, and inefficient process of convection ovens, so as to achieve efficient sterilization of antiseptic solutions and maintain the antiseptic
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
examples
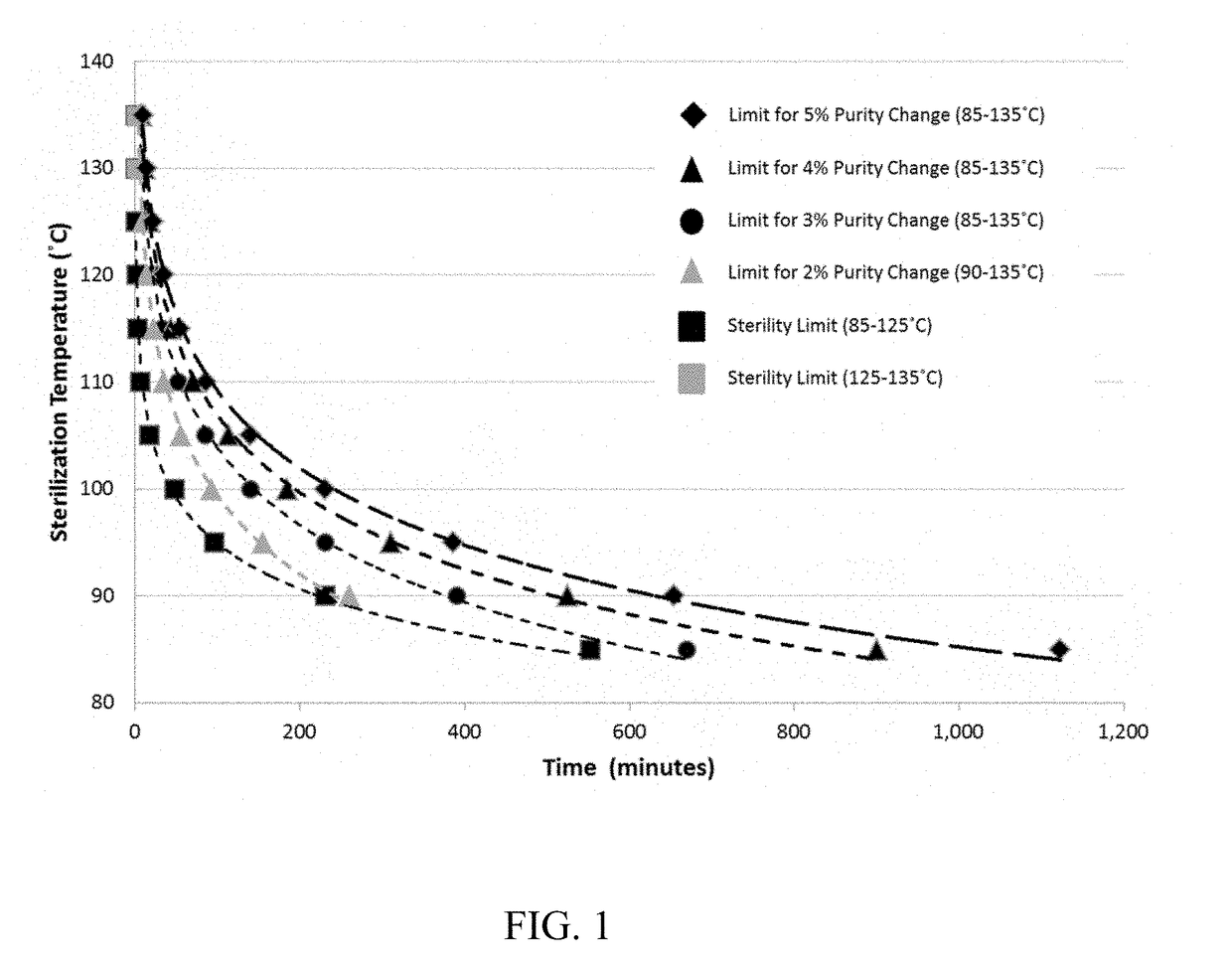
[0040]A sample of antiseptic solution of 70% v / v isopropanol, 30% v / v water, and 2.0% w / v chlorhexidine gluconate contained in a glass ampoule was tested in each of the following examples. An inoculum of greater than 1,000,000 but less than 10,000,000 test spores of Geobacillus stearothermophilus were inserted and sealed into the container. In the following examples, a 10 mL sample of antiseptic solution at room temperature was placed in either a water or oil bath (water bath for temperatures of ≤95° C.; oil bath for temperatures≥100° C.) having a preset temperature (i.e., the sterilization temperature). The ampoule containing chlorhexidine gluconate solution and test spores was placed in the heating medium. The sample with test spores was removed at a particular time (i.e., the sterilization time), allowed to cool to room temperature, then tested and incubated over a seven day period for bacterial growth. Samples of antiseptic solution also stored at the preset temperature were tes...
PUM
| Property | Measurement | Unit |
|---|---|---|
| temperature | aaaaa | aaaaa |
| time | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com