Methods for cancer therapy
a cancer therapy and cancer technology, applied in the field of cancer therapy, can solve the problems of multiple myeloma remaining incurable, bone marrow failure, hypercalcemia, renal failure, etc., and achieve the effect of preventing the progression of multiple myeloma
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
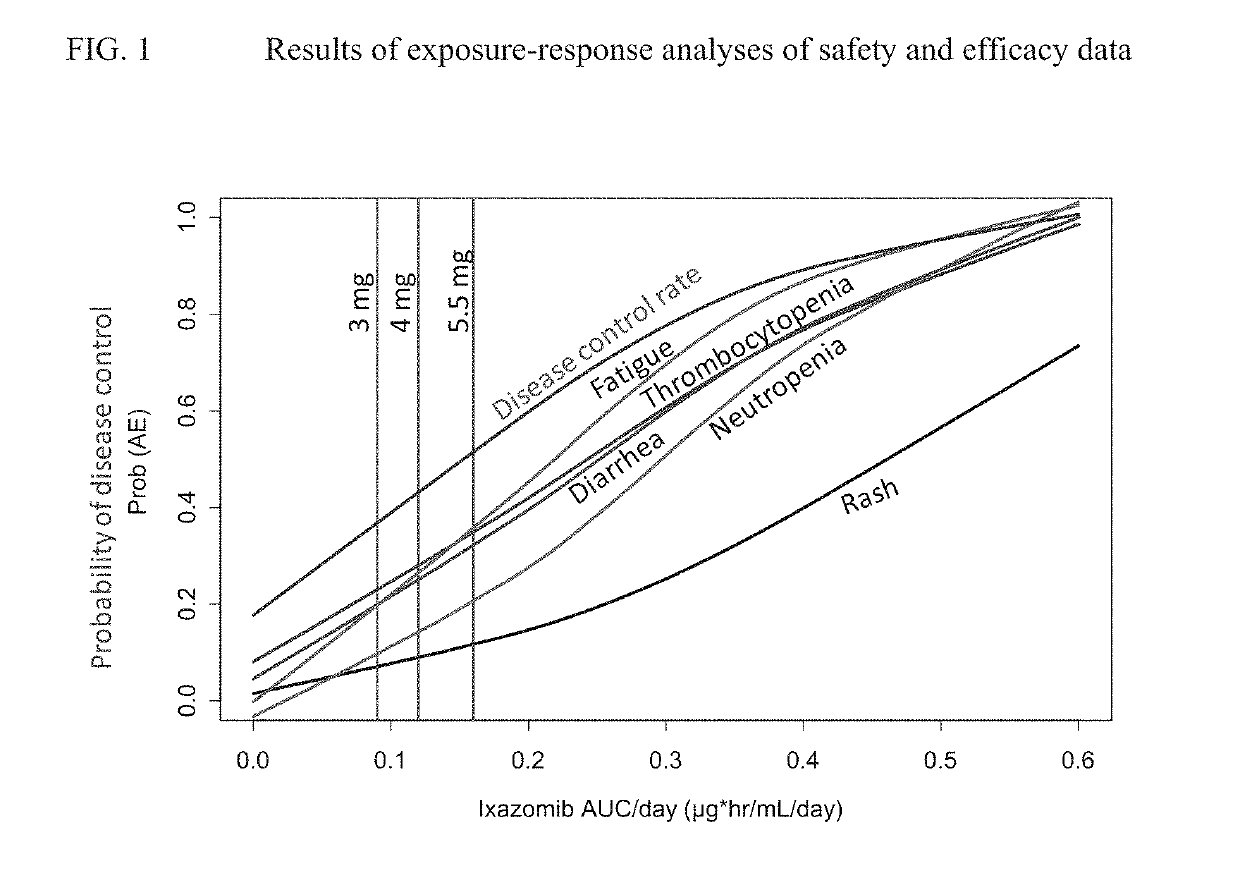
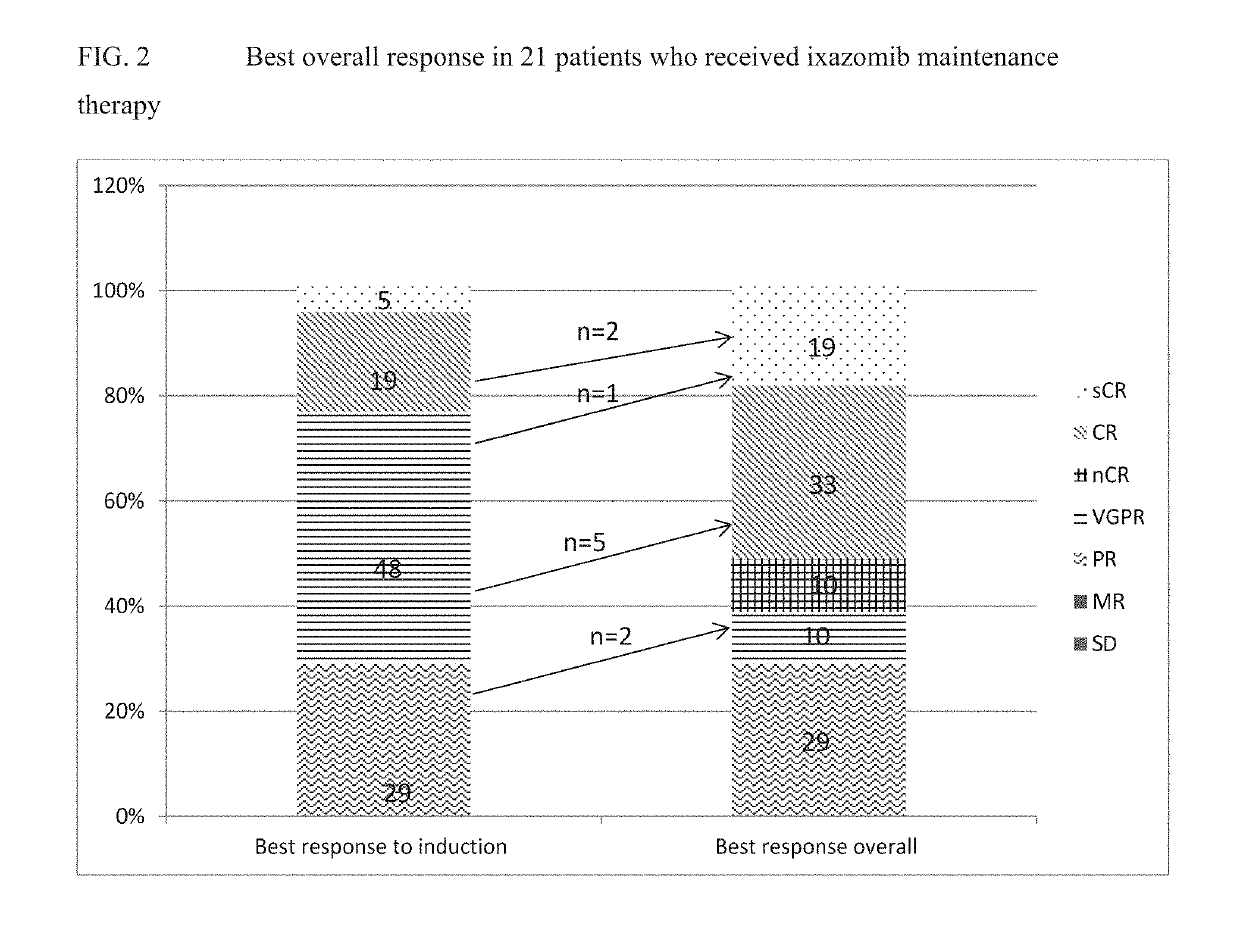
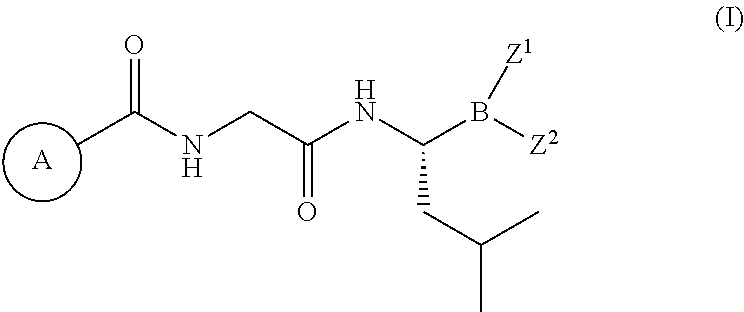
[0031]The present disclosure provides various methods for treating cancer, or preventing cancer recurrence or progression. In the first aspect, the disclosure provides administering to a cancer patient a compound of formula (I), or a pharmaceutically acceptable salt thereof. In another aspect, the disclosure provides a dosing schedule of a compound of formula (I), or a pharmaceutically acceptable salt thereof, as a maintenance therapy to a patient who has undergone a primary cancer therapy and responded. In a further aspect, the disclosure provides maintenance therapy comprising a proteasome inhibitor of formula (IIIa), or a pharmaceutically acceptable salt thereof, to improve and maintain response for patients who have undergone a primary cancer therapy.
[0032]In another aspect, the disclosure provides maintenance therapy comprising a proteasome inhibitor of formula (IIIa), or a pharmaceutically acceptable salt thereof, to prevent patients from cancer recurrence or progression.
[0033...
PUM
| Property | Measurement | Unit |
|---|---|---|
| time | aaaaa | aaaaa |
| time | aaaaa | aaaaa |
| time | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com