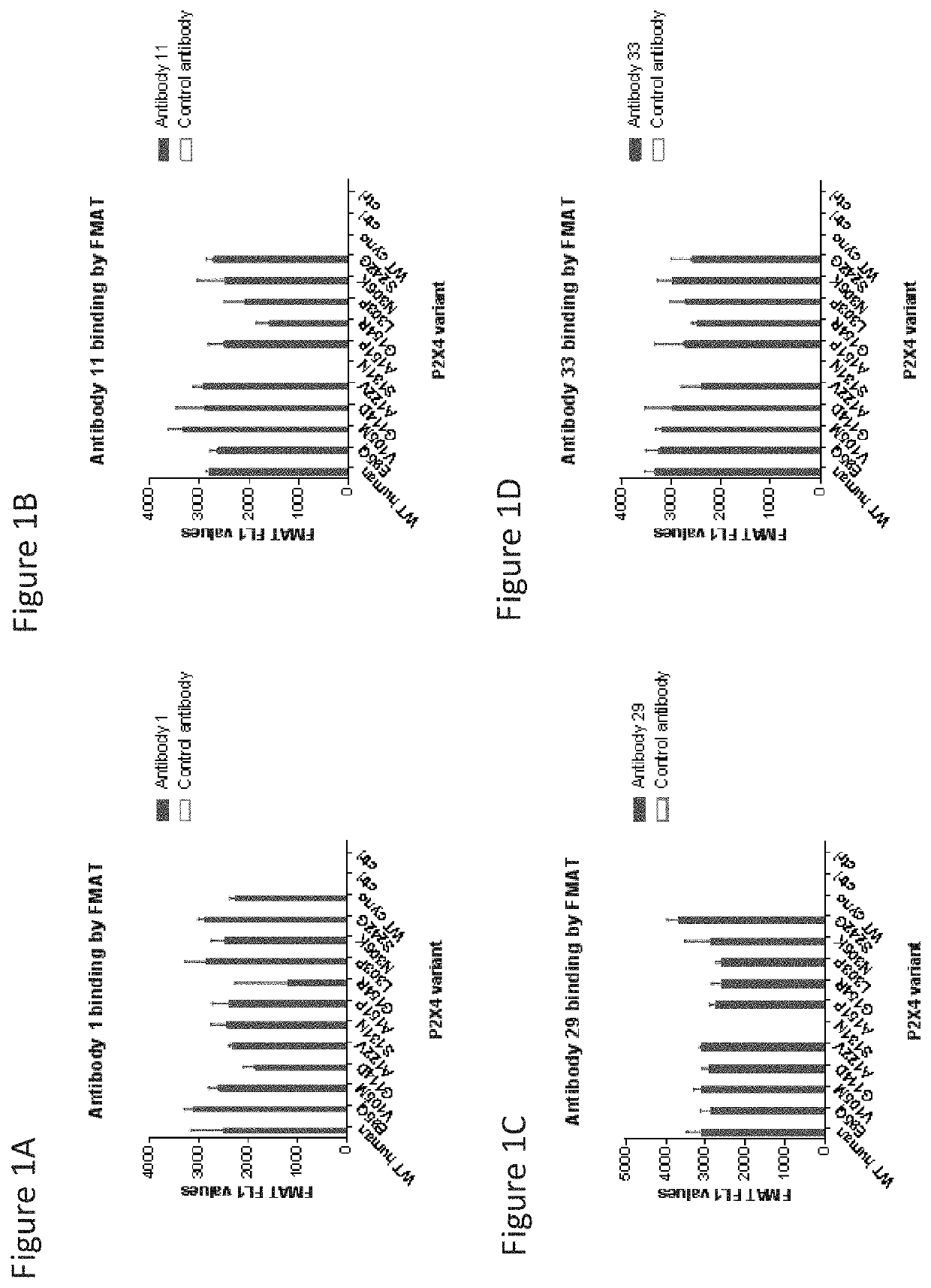
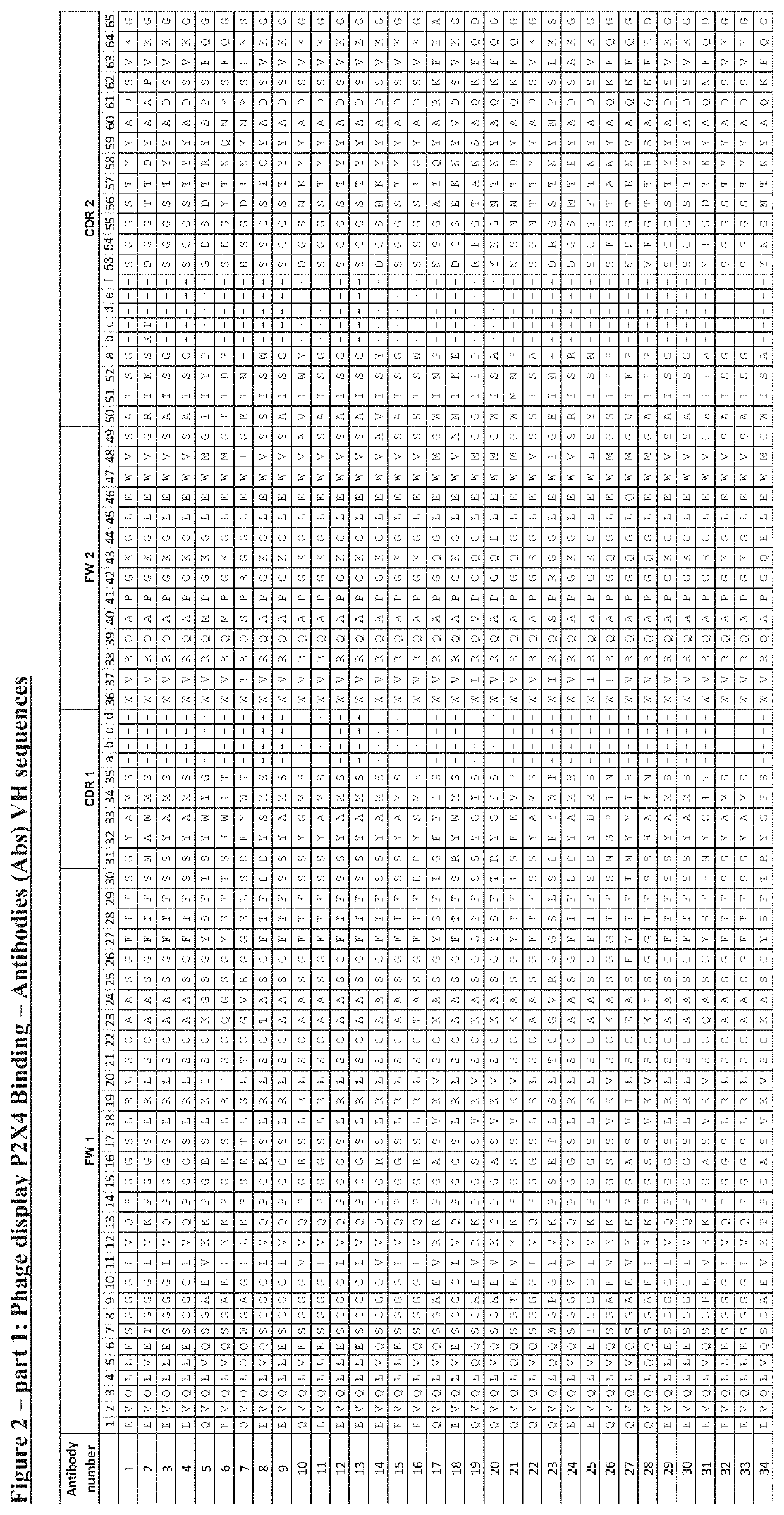
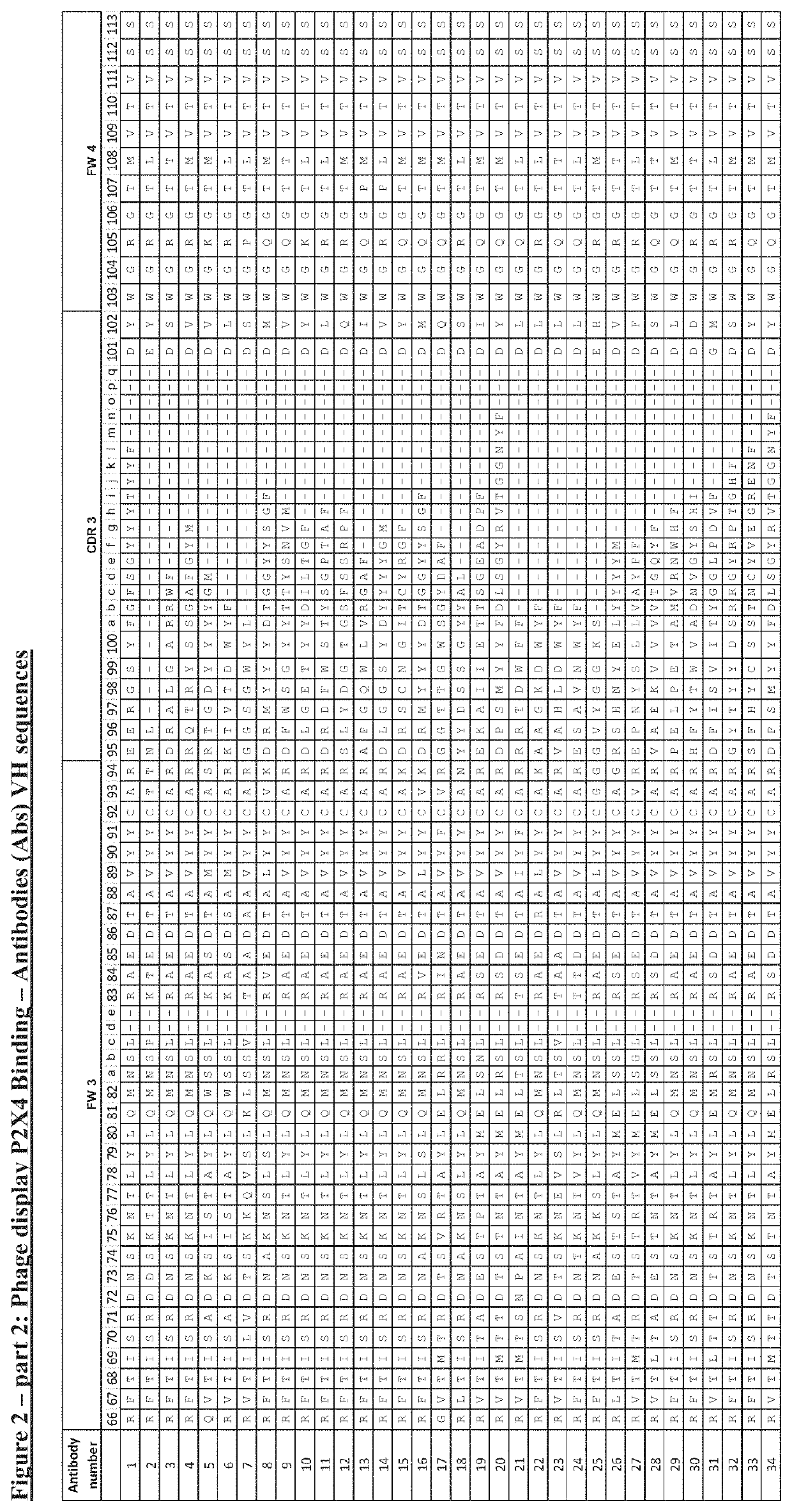
P2x4 antibodies & uses thereof
a technology of p2x4 antibodies and antibodies, applied in the field of p2x4 antibodies, can solve the problems of neuropathic pain not responding to currently available analgesics, no beneficial purpose of chronic pain, etc., and achieve the effects of reducing or eliminating antibody binding, modulating channel activity, and reducing p2x4 biological activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
n and Isolation of Recombinant P2X4 Proteins
[0167]Human P2X purinoceptor 4 (Q99571), a natural variant of human P2X purinoceptor 4 with an S to G mutation at position 242 (Corresponds to variant rs25644) and murine P2× purinoceptor 4 (Q9JJX6) proteins were designed with a C-terminal AVI tag (Avidity LLC) and a C-terminal Histidine tag. The constructs were cloned into pFASTBAC1 vectors (Life Technologies). Bacmids were generated in DH10Bac (Life Technologies) E. coli cells. Bacmids were subsequently transfected into Sf9 insect cells (Spodopterafrugiperda Sf9 cells from Life Technologies, cat no 11496-015) for production of recombinant baculovirus particles, which in turn were used to infect Sf9 cells for protein expression.
[0168]Expression parameters were assessed by monitoring expression level, protein quality and the homogeneity of the receptor using a modified Fluorescence-detection size-exclusion chromatography (FSEC) method described by Backmark et al., (Protein Sci. 22 (2013) 1...
example 2
ion of Trimeric P2X4 Complexes
[0171]In vivo, P2X receptors form functional trimeric ion channels. The solubilised and purified P2X4 proteins are typically present in a range of oligomeric states, including monomers, dimers, trimers, and hexamers (i.e., dimers of trimers). This range of oligomeric states is described for example, by references (Backmark et al., Protein Sci. 22 (2013) 1124-1132; Kawate et al., Structure 14 (2006) 673-681; Kawate et al., Nature 460 (2009) 592-598; Nakazawa et al., European Journal of Pharmacology 518 (2005) 107-110; Nicke et al., Mol. Pharmacol. 63 (2003) 243-252). To obtain a stable predominantly trimeric arrangement, solubilization conditions were adjusted.
[0172]Combinations of detergents, additives, buffers and pH were varied. Optimal conditions were selected to increase the FSEC signature of the trimer while reducing larger order oligomeric arrangements and aggregates. Such undesirable forms were eluted in the void volume of the size-exclusion colu...
example 3
Specific Antibodies were Isolated Using Phage Display Selection
[0173]Naïve human single chain Fv (scFv) phage display libraries were cloned into a phagemid vector based on the filamentous phage M13 were used for selections (Lloyd (2009) Protein Eng Des Sel 22, 159-168; Vaughan et al., Nature biotechnology 14, 309-314, 1996). Anti-P2X4 specific antibodies were isolated from the phage display libraries using a series of selection cycles on recombinant human P2X4 (hu P2X4), essentially as previously described by Vaughan et al (Vaughan et al., supra). In brief, human P2X4 in PBS (Dulbecco's PBS, pH7.4) was immobilised onto wells of a MaxiSorpL microtitre plate (Nunc) overnight at 4° C. Wells were washed with PBS then blocked for 1 hour with PBS-Marvel dried skimmed milk (3% w / v). Purified phage in PBS-Marvel (3% w / v) were added to the wells and allowed to bind coated antigen for 1 hour at room temperature. Unbound phage was removed by a series of wash cycles using PBS. Bound phage parti...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com