Method and composition for treating inflammatory bowel disease
a technology for inflammatory bowel disease and composition, applied in the field of method and composition for treating inflammatory bowel disease, can solve the problems of limiting cell migration into the matrix, adverse immune responses, and none of the above-mentioned problems in the treatment or regeneration of lower intestine tissue, and achieve the effect of preventing the onset of rectal mucositis
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Surgical Placement of Solid ECM for Replacement of Colon Tissue
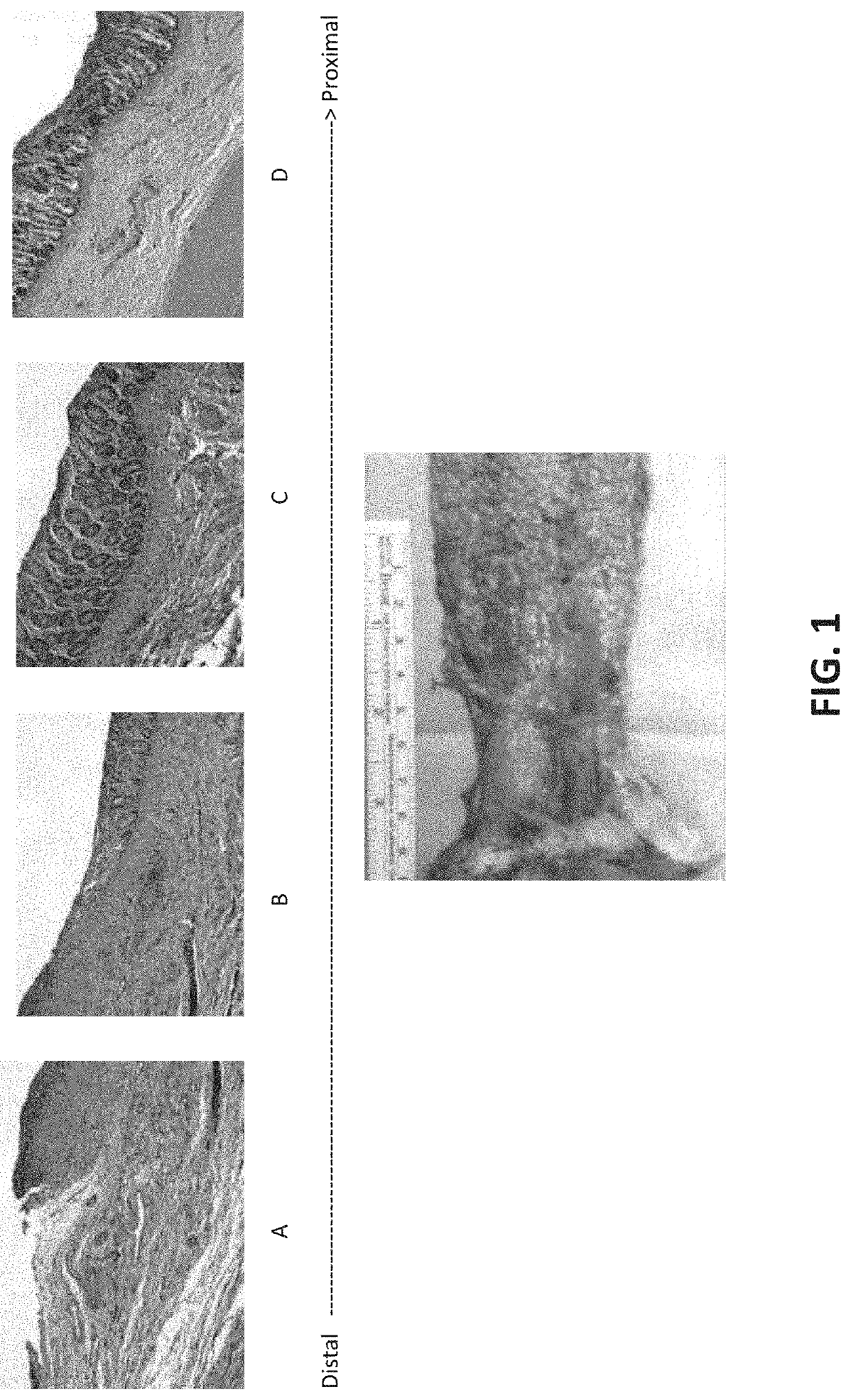
[0132]Small intestine submucosal extracellular matrix (SIS-ECM) grafts were grafted into the colon tissue of four (4) living dogs. The SIS-ECM grafts of approximately 3-4 centimeter lengths were prepared in accordance with known techniques. Grafts were prepared having different numbers of layers—2-layer, 4-layer, 6-layer, and 8-layer sheets or tubes and tested following transanal circumferential mucosal resection.
[0133]Four (4) healthy adult mongrel female dogs (approx. 20 kg in weight) were subjected to circumferential colon mucosal EMR. With the dog under general anesthesia, surgical mucosectomy was performed. Alternatively, mucosal tissue ablation, such as EMR can be performed, e.g., with a therapeutic endoscope (EG-3430, Pentax Medical, Montvale, N.J.) and a commercially available kit (EMR Kit, Olympus America, Center Valley, Pa.). Piecemeal mucosal resections were sequentially performed until a 4-cm circumferential ...
example 2
Application of Gel ECM for Replacement of Colon Tissue
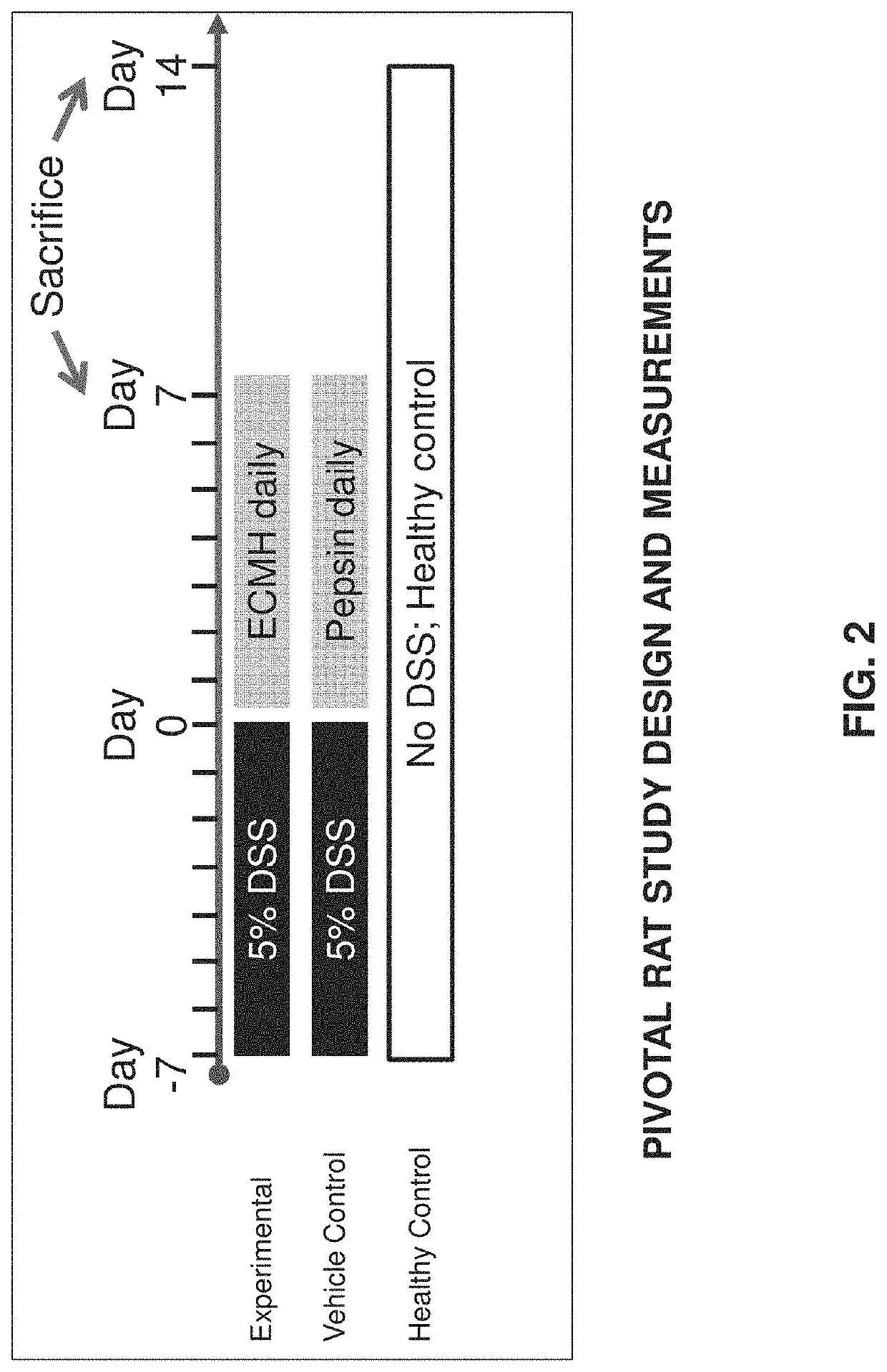
[0150]In accordance with the invention, the feasibility of using an ECM gel composition for treatment of Inflammatory Bowel Disease (IBD) can be tested in vivo in rats. Rats may be established as a model for human IBD, such as Ulcerative Colitis (UC), whereby a UC-like inflammation can be induced in rat colon tissue by administering Dextran Sulfate Sodium (DSS) in drinking water ad libitum for several days.
[0151]Acute UC-like inflammation can be induced in rats by administering, in drinking water provided ad libitum, 5% DSS for seven days, followed by followed by administration of regular water. Chronic UC-like inflammation can be induced in rats by administering, in drinking water provided ad libitum, 5% DSS for one or more consecutive 7-day periods, followed by administration of regular water.
[0152]Treatment of UC can be carried out using a gel extracellular matrix (ECM) derived from small intestine submucosa (SIS) and introduc...
example 3
In Vivo Study Using ECM Hydrogel (ECMH) For Remodeling of Colon Tissue in Rats
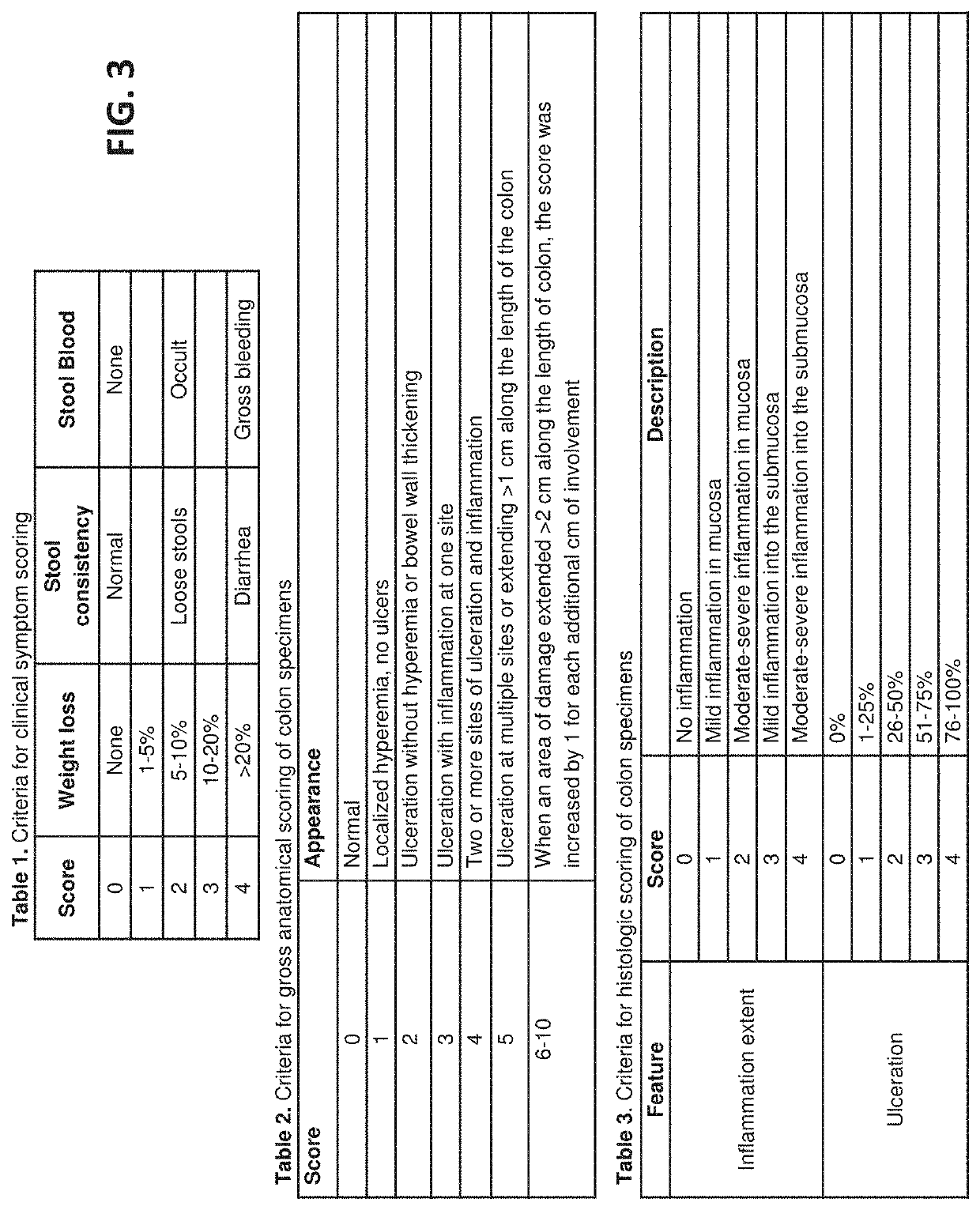
[0178]A nonsurgical and nonpharmacological approach to UC therapy can abate inflammatory flares by promoting alternative activation of the local innate immune cell population, and induce rapid replacement of colonic mucosal barrier function by restoring mucosal epithelial structure. The present study demonstrates efficacy of local delivery of a hydrogel form of mammalian extracellular matrix (ECMH) for treating UC. The effect of ECMH on clinical symptomology, inflammation, and epithelial barrier function was evaluated by multiple in-vivo and in-vitro outcome measures.
[0179]The study, presented in this Example, shows that an enema hydrogel composed of ECM effectively treats a rodent model of UC. Effective therapy was determined according to two essential physiologic processes that were positively directed by ECMH treatment: 1) restoration of colonic epithelial barrier function, which protects the host from ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| residence time | aaaaa | aaaaa |
| residence time | aaaaa | aaaaa |
| residence time | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com