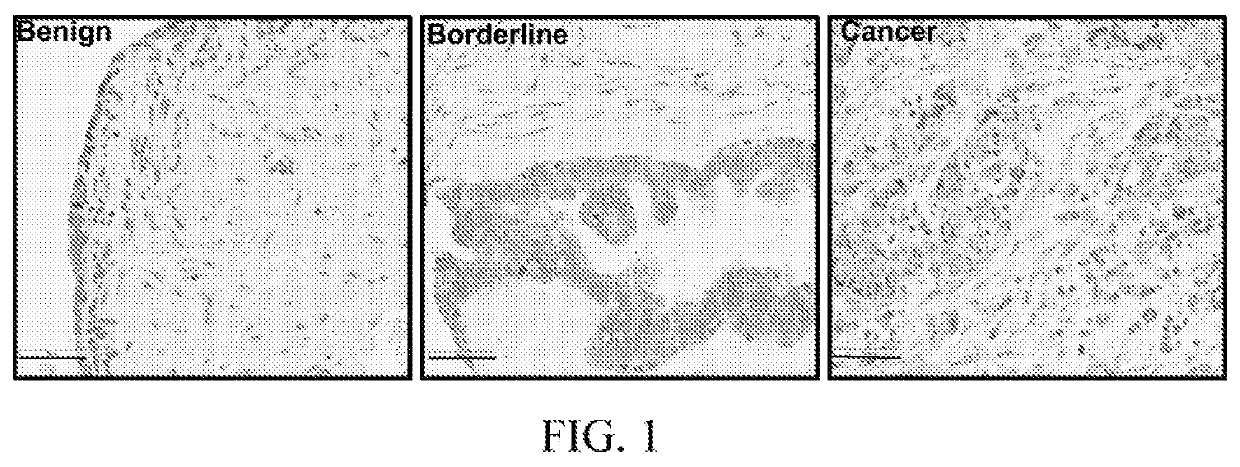
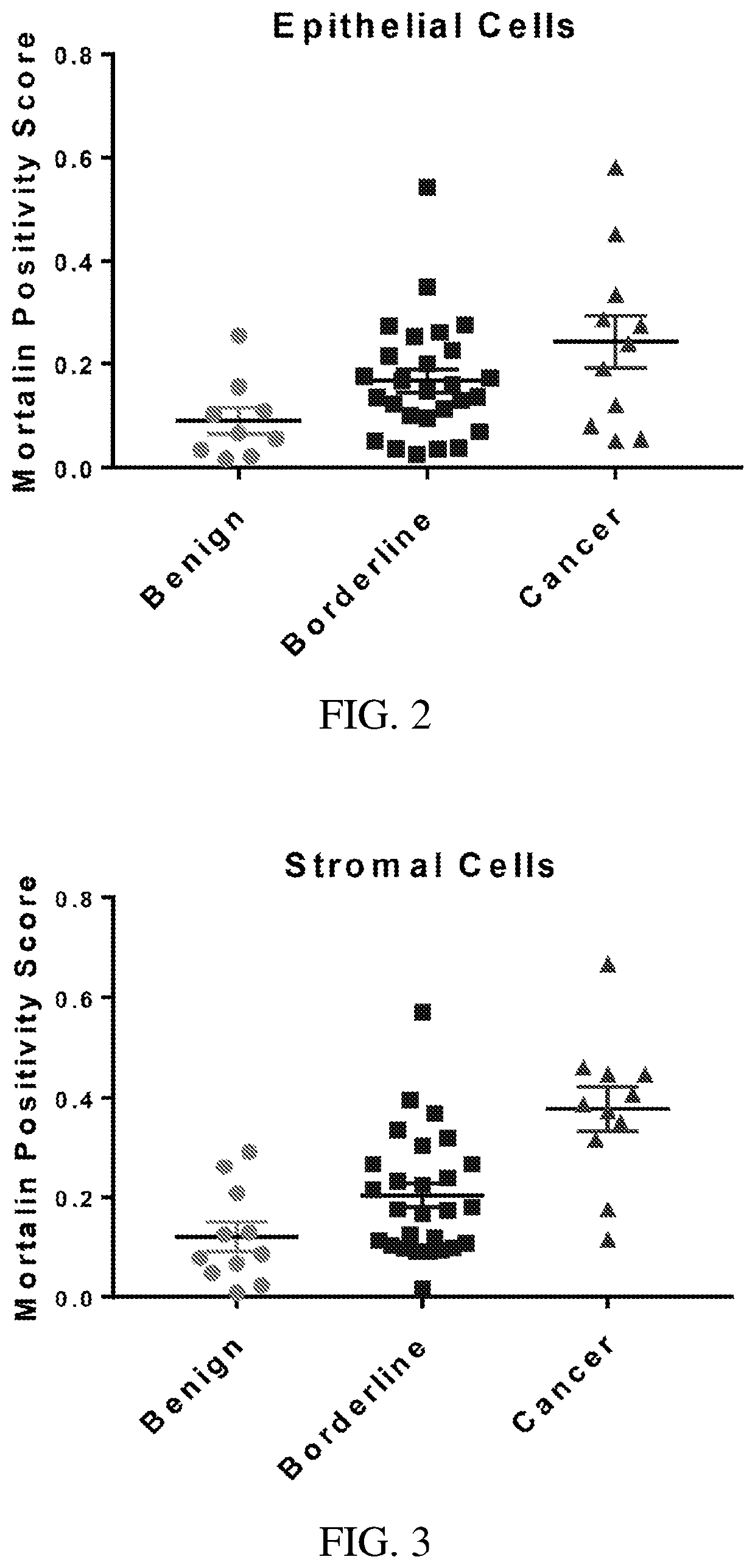
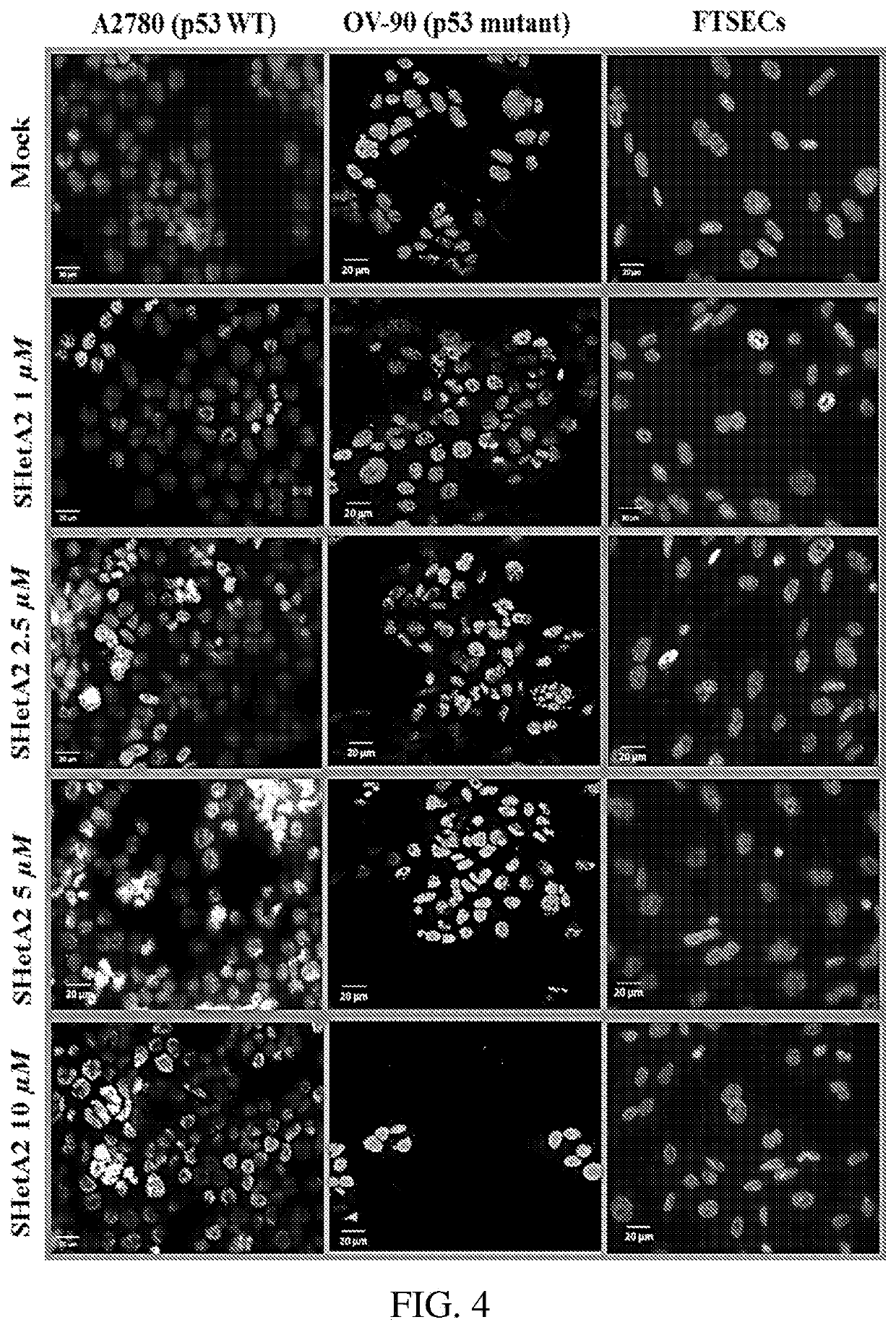
Combination cancer therapies
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
SHetA2—PRIMA-1 / PRIMA-1MET Drug Combinations
Methods
[0149]Cell Lines, Plasmids and Drugs
[0150]Ovarian cancer cells lines A2780 (RRID:CVCL_0134), OV-90 (RRID:CVCL_3768), SKOV3 (RRID:CVCL_0532), COV362 (RRID:CVCL_2420), OVSAHO (RRID:CVCL_3114), Caov3 (RRID:CVCL_0201), and OVCAR4 (RRID:CVCL_1627) (all from American Type Culture Collection / ATCC Manassas, Va., USA) were cultured in RPMI1640 medium containing 10% FBS and 1×antibiotic / antimycotic. The MESOV / GFP-luc (RRID:CVCL_CZ92) cell line (gift from Dr. Francois Moisan, Stanford University) (MESOV) was cultured in DMEM-high glucose medium with 10% FBS. SKOV3 cells stably transfected with mutant p53 (R248W, R273H) or parent vector were provided by Jeremy Chien, PhD (University of Kansas Medical Center). Human fallopian tube secretory epithelial cells (hFTSECs) were isolated from fallopian tube fimbriae surgical specimens under an IRB approved protocol. All cell lines were authenticated by autosomal short tandem repeat (STR) profiles determ...
example 2
SHetA2—Palbociclib Drug Combination
[0198]CDK 4 / 6 inhibitors (e.g., Palbociclib, Ribociclib and Abemaciclib) are structurally-related drugs currently FDA approved for treatment of cancer. As a demonstration that SHetA2 works synergistically with the CDK 4 / 6 group of drugs, SHetA2 was tested for its ability to interact synergistically with palbociclib.
[0199]Palbociclib, an FDA approved anticancer drug for treatment of breast cancer, was evaluated in combination with SHetA2 because they inhibit cyclin D1 / CDK4 / 6 complex-driven cancer cell growth through complementary mechanisms. Palbociclib binds and inhibits the CDK4 / 6 kinase activity. SHetA2 disrupts mortalin complexes and mortalin is known to bind cyclin D1. Another SHetA2 binding target, Hsc70, stabilizes the cyclin D1 / CDK4 / 6 complex. Furthermore, SHetA2 causes degradation of cyclin Dl in cell lines, and in animal models in association with tumor reduction.
[0200]Strong synergy was observed between SHetA2 and Palbociclib when used to...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Dimensionless property | aaaaa | aaaaa |
| Dimensionless property | aaaaa | aaaaa |
| Composition | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com