MUC1 PARALLEL CAR (pCAR) THERAPEUTIC AGENTS
a technology of parallel cars and therapeutic agents, applied in the direction of drug compositions, peptides, blood/immune system cells, etc., can solve the problems of disappointing third-generation cars, limited efficacy of t cells, and insufficient engagement of cd3z-chain fusion receptors to elicit substantial il-2 secretion and/or t cell proliferation, etc., to achieve effective t cell responses
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1a
ctivity of pCAR-T Cells
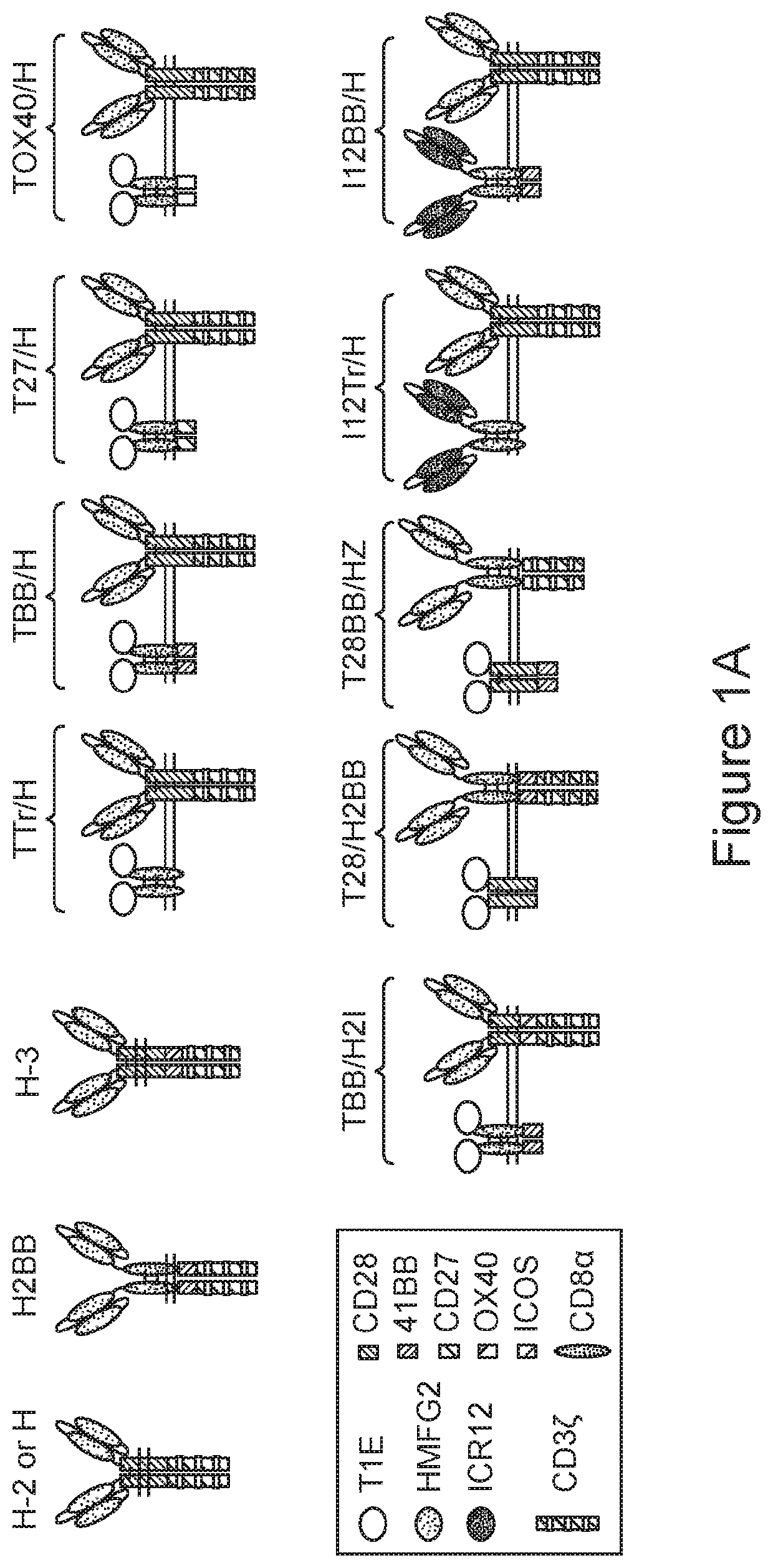
[0326]Untransduced (UT) T cells and T cells expressing the 2nd generation H CAR, TTr / H pCAR, TBB / H pCAR, I12Tr / H pCAR and I12BB / H pCAR respectively were co-cultivated in vitro for 72 hours with MDA-MB-468 breast cancer cells. The effector:target (T cell:tumor cell) ratio ranged from 2 to 0, including 2, 1, 0.5, 0.25, 0.125, 0.06, 0.03 and 0. Residual viable cancer cells after the co-culture were quantified by MTT assay. The percentage survival of MDA-MB-468 breast cancer cells after co-culture with the CAR-T cells or pCAR-T cells is presented in FIGS. 2A and 2B.
[0327]MDA-MB-468 breast cancer cells express both MUC-1 and ErbB dimers with very low level of HER2. As shown in FIG. 2A, at the effector:target ratio of 0.5, H CAR-T cells showed clear cytotoxic anti-tumor activity compared to untransduced T cells. TTr / H pCAR-T cells and I12Tr / H pCAR-T cells were more effective than the H CAR-T cells in targeting MDA-MB-468 breast cancer cells, suggesting that the CCRs...
example 1b
ctivity of pCAR-T Cells
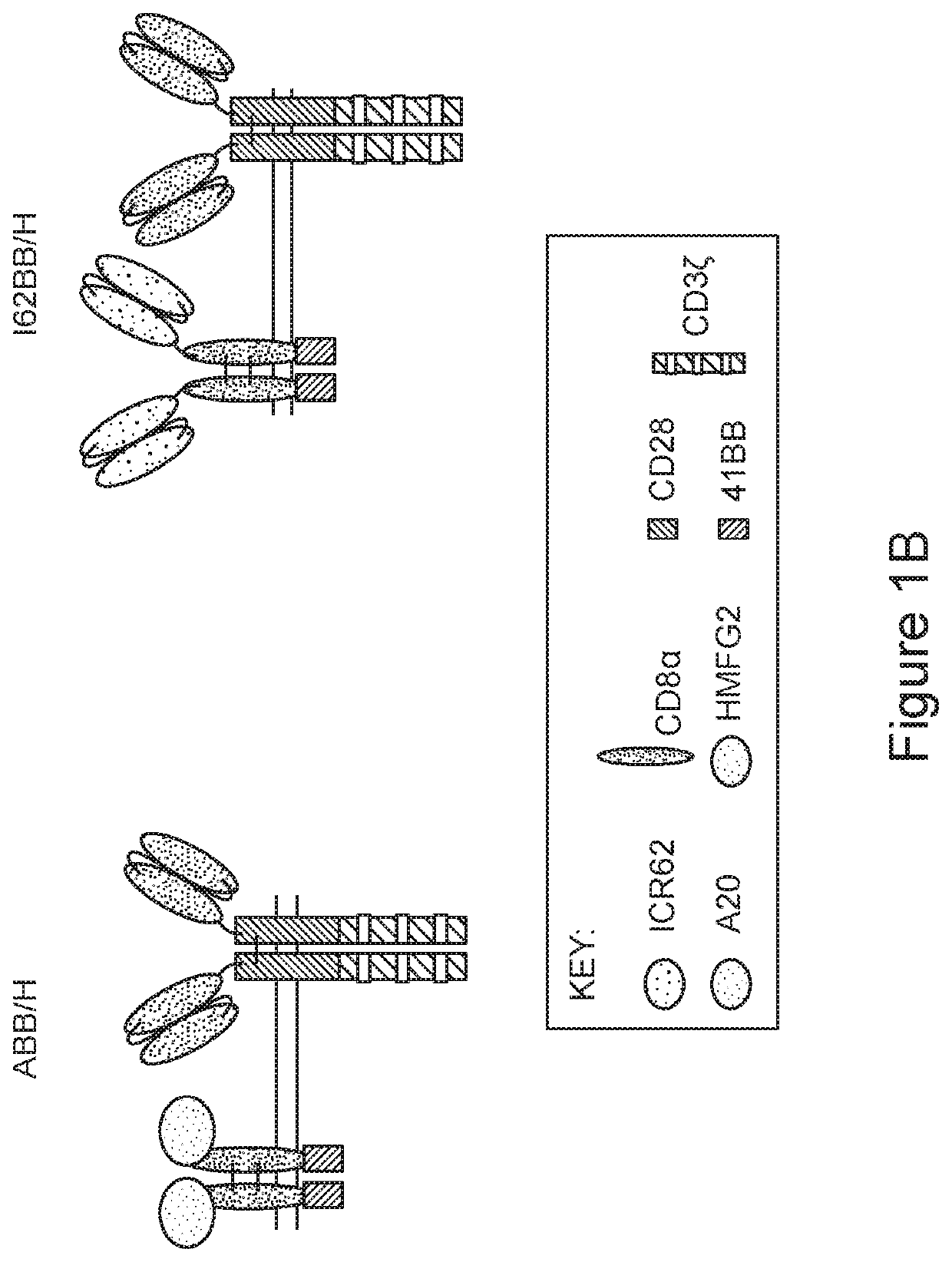
[0329]Untransduced (UT) T cells and T cells expressing the CARs or pCARs of FIGS. 1A and 1B were co-cultivated in vitro for 72 hours with MDA-MB-468 breast cancer cells at different ranges of effector:target (T cell:tumor cell) ratio. Residual viable cancer cells after the co-culture were quantified by MTT assay. The percentage survival of MDA-MB-468 breast cancer cells after co-culture with the untransduced T cells, CAR-T cells, or pCAR-T cells is presented in FIGS. 8A-C, 9 and 10.
[0330]As shown in FIG. 8A, TTr / H pCAR-T cells were more effective than H-2 CAR-T cells in targeting MDA-MB-468 breast cancer cells. As the CCR of TTr / H pCAR is truncated and lacking the 4-1BB co-stimulatory domain, the superior results of TTr / H compared to H-2 suggest that the truncated CCR improved the CAR-T function by a docking effect. TBB / H pCAR-T cells were more effective than TTr / H pCAR-T cells, suggesting that the CCR further improved the CAR-T function by the co-stimulatory ...
example 2a
e-Stimulation Potential of pCAR-T Cells
[0335]T cells that express CARs and pCARs were subjected to successive rounds of antigen (Ag) stimulation in the absence of exogenous cytokine IL-2. The engineered T cells were re-stimulated twice weekly with cancer cell monolayers.
[0336]H CAR-T cells and TTr / H and TBB / H pCAR-T cells were combined with MDA-MB-468 breast cancer cells at an effector:target ratio of 0.5 T cell:1 tumor cell, and tumor cell cytotoxicity was assessed twice weekly. CAR-T and pCAR-T cells progressed to a further round of antigen stimulation if more than 10% cytotoxicity was observed compared to tumor cells alone. The number of rounds of antigen stimulation was recorded. As shown in FIG. 4A, TTr / H pCAR-T cells exhibited increased re-stimulation potential as compared to the second generation H CAR-T cells. TBB / H pCAR further increased the re-stimulation potential of the engineered T cells as compared to TTr / H pCAR.
[0337]Engineered T cells expressing the CARs and pCARs we...
PUM
| Property | Measurement | Unit |
|---|---|---|
| nucleic acid | aaaaa | aaaaa |
| resistance | aaaaa | aaaaa |
| transforming growth factor-α | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com